The rising rates of dyslipidemia, type 2 diabetes, cardiovascular disease, and other metabolic conditions in the United States constitute a major public health crisis requiring a robust and effective multistakeholder response (
1,
2). The risk of developing these metabolic conditions is higher for people with schizophrenia (
3,
4), and antipsychotic drugs, the mainstay of schizophrenia care, contribute to this excess risk. The strength of the evidence for the association between second-generation antipsychotics, the most widely used class of antipsychotics, and metabolic risk varies substantially by drug (
5–
12). However, the high metabolic risk associated with several of these drugs is incontrovertible (
13–
16).
Although several professional societies have called for the routine assessment of glucose and lipid blood levels and other indicators of metabolic health to guide prescribing of second-generation antipsychotics (
17,
18), antipsychotic-treated Medicaid populations are infrequently tested for metabolic abnormalities (
19–
21).
Because Medicaid is the most common payer for the treatment of nonelderly adults with schizophrenia (
22), state Medicaid programs have a strong incentive to improve the safety of antipsychotic prescribing for this population. Although metabolic testing generates upfront costs associated with testing and treatment of conditions thus detected, metabolic testing may reduce this preventable morbidity and also reduce long-term health care costs. Therefore, efforts to increase testing rates may have important health and economic impacts.
Medicaid programs and the managed care organizations with which they contract may adopt a variety of policies (for example, pay for performance [P4P]) and other strategies (for example, academic detailing) to increase testing rates among beneficiaries with schizophrenia. Decision makers weighing whether to adopt any such policies often have incomplete information on policy benefits and costs. Simulation models can assist with policy design and implementation by providing preliminary information on the likely impacts of policies.
We conducted a microsimulation study to provide Medicaid policy makers and other stakeholders with information on the potential effects of policies aimed at increasing metabolic testing rates among beneficiaries with schizophrenia receiving antipsychotics. Our outcomes were ten-year prevalence rates of diabetes and prediabetes, a diagnosable precursor of diabetes, and their associated health care costs. We focused on diabetes because of its high public health significance.
Methods
Data Sources and Study Population
We used California Medicaid Analytic eXtract data for calendar years 2002–2009. California has the largest and one of the most diverse Medicaid populations, an important consideration because diabetes risk varies by race-ethnicity. We identified fee-for-service, non–dual-eligible, continuously enrolled Medicaid beneficiaries with schizophrenia diagnoses (ICD-9 codes 295.xx) recorded in two or more outpatient claims (primary or secondary) or one or more inpatient claims during a 12-month period and observed their antipsychotic prescription fills from the date of the first schizophrenia diagnosis claim through December 31, 2008 (to allow for 12 months of data through the end of 2009; N=61,469). We excluded beneficiaries ages 0–19 and over age 65 (N=2,266) and with race-ethnicity other than black, Latino, and white, because only these categories were available in our other data sources (N=10,221).
Beneficiaries meeting the above criteria (N=48,982) contributed one to seven person-years to a panel data set containing 12-month aggregated information on antipsychotic utilization (drug and its assigned metabolic risk), metabolic testing, diabetes conditions (a term hereafter used to refer to prediabetes and diabetes), and Medicaid spending. We used this panel data set to calculate several simulation model parameter estimates (for example, transition probabilities for diagnosed diabetes and prescription drug utilization and transition rates) as described below. [Additional details are included in an online supplement to this article.]
We defined beneficiaries from the panel data set with 2002 observations as the “simulation cohort” (N=21,491) and used these beneficiaries and their 2002 demographic data and initial antipsychotic prescribing and diabetes conditions as the baseline for the microsimulation.
We assigned each person-year to one of three metabolic risk groups: high if we observed more than a 90-day cumulative supply of clozapine, olanzapine, or a low-potency first-generation antipsychotic (for example, chlorpromazine); medium if we observed more than a 90-day cumulative supply of risperidone, quetiapine, or a medium-potency first-generation antipsychotic (for example, perphenazine); and low if we observed fewer than a 90-day cumulative supply of the aforementioned antipsychotics or more than a 90-day supply of aripiprazole, ziprasidone, or a high-potency first-generation antipsychotic (for example, haloperidol) in the one-year period. Drugs were classified on the basis of the literature (for example,
13–
16) and the expertise of a member of our research team (JN) to classify drugs. Because there is insufficient evidence about the impact of antipsychotic combinations on metabolic risk (for example, whether risks are additive), polypharmacy regimens were assigned the risk of the highest-risk drug in the combination. We calculated exposure with the fill date and days supplied variables. We operationalized metabolic testing as receipt of at least one lipid test and at least one glucose test during a 12-month period [see
online supplement for the Current Procedural Terminology codes]. Testing rates were calculated separately for individuals with and without a diabetes diagnosis.
As in other claims-based research (
20), we identified beneficiaries with diagnosed diabetes as those with one or more inpatient or two outpatient claims with a diagnosis of diabetes or complications (
ICD-9 codes 250, 357.2, 362.0, and 366.41). Claims-based diabetes diagnoses may be an underestimate of actual prevalence because of underdocumentation of diagnosed prediabetes or underdetection of both conditions. We overcame this limitation by using information on diagnosed prediabetes and undiagnosed diabetes conditions for black, Latino, or white individuals ages 20–64 available in the National Health and Nutrition Examination Survey (NHANES) data from 2007 to 2010. Because persons with schizophrenia may differ from the general population regarding both the risk of diabetes and its demographic distribution, we adjusted NHANES estimates with factors derived from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) (
11,
23) [see
online supplement for details].
The study was approved by the RAND Institutional Review Board.
Simulation Model
We developed a microsimulation model of prescriber decision making and disease progression over a ten-year horizon. In each iteration of the model, we simulated ten annual periods starting in 2002 through 2012 for the simulation cohort.
Model inputs.
Our inputs included demographic characteristics (age; sex; and white, black, or Latino race-ethnicity), rates of undiagnosed and diagnosed diabetes conditions, rates of incident diabetes conditions, antipsychotic exposure over time in the three risk categories, prior metabolic testing, prescriber decision parameters (that is, how prescribers interpret and respond to test results), and policy effectiveness. Only demographic variables, initial antipsychotic risk category, and initial rates of undiagnosed and diagnosed diabetes conditions were assessed at baseline. [Detailed descriptions of each simulation parameter are included in the online supplement.]
Because policy effectiveness varies widely depending on context (for example, type of delivery system) and program design (for example, size of incentive payments) (
24,
25), we let it vary from 0%, or status quo testing rates (that is, observed testing rates in the Medicaid data), to 100%, a universal-testing scenario in which every eligible patient is tested annually. Because a 20% improvement in testing rates is feasible with modest policies (for example, with P4P programs incentivizing screening [
26]), the model allowed for 20% increments from the status quo to the universal-testing scenario. Readers must judge whether larger impacts are feasible in their specific context.
Model outcomes.
Our outcomes included metabolic testing rates (the proportion of years during which individuals received metabolic testing); rate of diabetes conditions diagnosed at the end of the study’s ten annual simulation periods (the proportion of diagnosed individuals relative to all individuals with diabetes conditions); years with diabetes conditions (a count of person-years with diabetes conditions regardless of diagnosis status); time to diagnosis (years from onset to diagnosis for individuals with incident diabetes conditions); and short-term costs (costs of person-level testing, and other health care costs over the ten-year period), discounted to the baseline year (2002) [see online supplement for details].
Simulation Model Framework and Evaluation
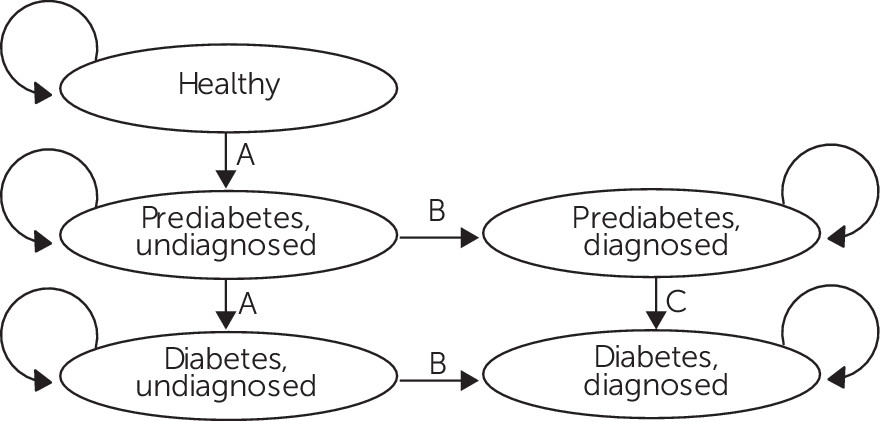
In each of the ten annual simulation periods, individuals were in one of five states: healthy (no diabetes conditions), undiagnosed prediabetes, diagnosed prediabetes, undiagnosed diabetes, or diagnosed diabetes (
Figure 1). The microsimulation allowed for individuals to transition to other undiagnosed health states as described in
Figure 1 but did not allow anyone to die or leave Medicaid.
We developed a set of assumptions for model inputs with insufficient empirical evidence. First, medium- and high-risk antipsychotics increase the risk of transitioning to diabetes condition states (that is, developing diabetes conditions) (
27). Second, testing rates do not depend on the antipsychotic prescribed. Third, test results reveal all diabetes conditions to the prescriber without error (that is, testing causes individuals to transition from undiagnosed to diagnosed states) (
Figure 1, label B). Fourth and last, test results revealing diabetes conditions lead prescribers to switch patients on medium- and high-risk drugs to a lower-risk drug 100% of the time. Although prescribers may prefer to implement an adjunctive intervention, such as metformin, over switching antipsychotics, our third assumption is supported by empirical evidence that had just began to emerge (
28,
29) when the most recent Schizophrenia Patient Outcomes Research Team treatment recommendations were issued (
30). Two randomized controlled trials (
31,
32) and two reanalyses of data from the CATIE study (
33,
34) have provided additional supporting evidence. This evidence indicates that although a switch from higher- to lower-risk antipsychotics improves metabolic indices, a significant loss of clinical benefit is unlikely unless the drug is clozapine, thus suggesting that a switch to lower-risk drugs should be considered a first-line strategy for patients who experience metabolic effects while taking nonclozapine antipsychotics. The risk-benefit analysis differs for clozapine, an underused drug in the United States (
35), given its unrivaled effectiveness for treatment-resistant and suicidal presentations (
30).
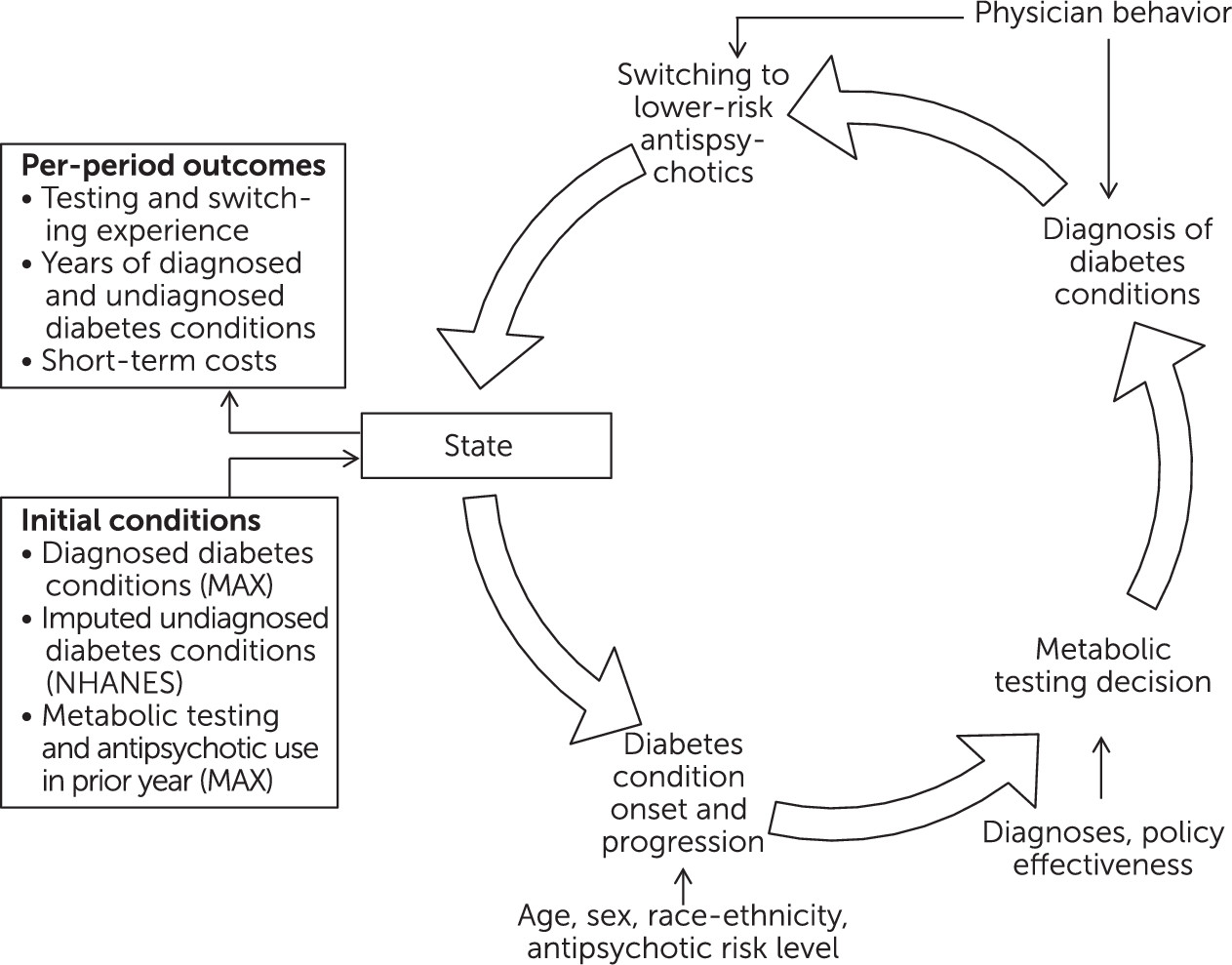
Figure 2 presents a graphical representation of our model.
We evaluated the simulation model in Stata 13. For our main results, we evaluated the model 1,000 times for each 20% increment in policy effectiveness.
Discussion and Conclusions
Using a microsimulation model, we found that hypothetical policies to incentivize metabolic testing have the potential to accelerate the diagnosis and reduce the prevalence of diabetes over a ten-year time horizon. This is an important finding because diabetes, a highly prevalent chronic illness, is associated with cardiovascular complications, disability, premature mortality, and substantial health care costs (
36). Although the size of the effect for some study outcomes was small—for example, a decrease of <1% in diabetes prevalence—even this small decrease may have important clinical and economic implications.
Policies that aim to reduce diabetes risk among antipsychotic-treated adults with schizophrenia by increasing testing rates must weigh implementation- and treatment-related costs relative to cost savings from preventing illness progression. We found that effective policies were associated with higher short-term costs. However, payers, patients, and the larger society might be willing to make the investment given the potential for better health outcomes. Although not assessed by our study, some of these outcomes may be evident only over a longer time horizon. These include improved cardiovascular health, improved mental health from better physical health, and improved quality of life.
Our study framed hypothetical policies in general terms rather than by specifying a particular intervention and its costs. We intentionally described our results in terms of relative policy effectiveness to increase generalizability across different strategies and health care contexts. A range of policies and other strategies may be implemented to promote safe and high-value care. These include quality improvement interventions, such as academic detailing and audit and feedback, value-based purchasing tools, value-based formulary management tools, and financial incentives (such as P4P). In this study, we focused on estimating the likely magnitude and direction of some of these impacts rather than on conducting a formal cost-effectiveness analysis of individual strategies. However, future research should simulate the cost-effectiveness of specific strategies implemented in specific health care settings. In this respect, it is illustrative that cost-effectiveness studies of diabetes screening in the general population suggest that screening is cost-effective among at-risk subgroups for whom costs are offset by lower future treatment costs (
37).
Although antipsychotics are a critical component of schizophrenia treatment, prescribers need to consider safety as well as “hidden” medium- and long-term costs of treatment. There is consensus on the importance of monitoring metabolic indices among patients prescribed antipsychotics, as recognized by the inclusion of a diabetes screening measure for antipsychotic-treated patients in the core set of adult quality measures for Medicaid populations (
38). However, despite notable efforts (
39), this practice remains underused and inconsistently incentivized in Medicaid (
20,
21).
Several factors contribute to the underuse of metabolic testing (
20). The fragmentation of the delivery and payment systems for publicly financed care of serious mental illness probably plays a key role, because the benefits of preventive interventions as well as the costs of their underuse spread over different actors and over time.
This study had some limitations. First, we relied on imputed NHANES data for undiagnosed diabetes and prediabetes prevalence rates, adjusted in aggregate to better approximate the population with schizophrenia. Although these health states are critical to decision makers who weigh the costs and benefits of policy options, they are unavailable in claims data. We were able to match rates for these conditions with the simulation cohort by using the demographic information available in the Medicaid data (including age range, sex, and race-ethnicity but not body mass index). Second, our simulations required a number of assumptions in regard to testing rates, prescriber behavior, and other parameter estimates. However, we based all assumptions on empirical evidence augmented with expert opinion provided by a member of our team (JN). Moreover, our results were robust to different assumptions, as demonstrated by our sensitivity analyses. Third, our study cohort included beneficiaries from only a single state, and thus results may not be generalizable elsewhere. Fourth, we assumed that individuals do not transition toward healthier states, although this is possible if diagnosis of a diabetes condition results in lifestyle or other changes, as a result we may have underestimated benefits from screening. Finally, because prediabetes diagnoses are inconsistently recorded in claims, we focused only on the incremental costs associated with diabetes diagnoses. As a result, we likely underestimated the total costs associated with diabetes conditions.