Opioid misuse is a significant public health problem. Estimates from the 2013 National Survey on Drug Use and Health show that nearly 1.9 million people in the United States have a substance use disorder related to prescription opioids and that 517,000 have a substance use disorder related to heroin (
1). The U.S. Centers for Disease Control and Prevention estimates that poisoning deaths involving an opioid analgesic accounted for 51.4% of all drug overdose deaths in 2013 (
2).
This epidemic has led to a significant increase in hospitalizations for opioid misuse (
3). Hospitalizations for opioid misuse increased by 150% between 1993 and 2012 (
4). In 2012, the hospitalization rate for opioid misuse was 295.6 stays per 100,000 (
4). Past research has shown that outpatient treatment services that include residential treatment, partial hospitalization, and intensive outpatient services are consistently associated with positive recovery and that patients who do not receive follow-up services have a much higher risk of being readmitted (
5–
7).
The U.S. Food and Drug Administration (FDA) has approved three different medications to treat opioid dependence: methadone, naltrexone, and buprenorphine (
8). When given through an individualized treatment regimen by a certified provider who has assessed the patient, these medications help reduce problem addiction behaviors (
8). The approved medications provide a safe, controlled level of medication to address the physiological effects of opioid addiction and can be safely administered for as long as needed. They are recommended as clinical treatment of adults who have an opioid use disorder with physiological dependence: they significantly augment treatment retention, decrease illicit opioid use, reduce the burden of opioid craving, and, in the case of agonist therapies, provide effective relief from opioid-withdrawal syndrome (
6,
9,
10).
The overarching goal of this study was to identify patterns of postdischarge prescription fills following a hospitalization for opioid misuse, a key step in determining whether patients are receiving appropriate medication after opioid-related inpatient treatment. Given the increasing rate of such hospitalizations, this timely information is important for developing targeted prevention, intervention, and treatment options for patients with opioid use disorders.
Methods
This study used data from the Truven Health MarketScan Commercial Claims and Encounters database for the years 2010 through 2014. The MarketScan database includes insurance claims from employees and their dependents covered by large, self-insured employers and by regional health plans. It captures all billed services, including prescription drugs, outpatient services, and inpatient services. Mental health and substance misuse services provided by separate behavioral health management companies through a carve-out arrangement also are captured in the database.
The cohort included individuals ages 18 to 64 who were hospitalized for opioid abuse, dependence, or overdose between January 1, 2010, and September 30, 2014, in an acute care hospital or psychiatric facility. Patients who did not have at least 30 days of continuous enrollment after being discharged were omitted to ensure that patterns of care after hospitalization could be identified. We also excluded individuals who did not have at least 90 days of continuous enrollment before their inpatient admission. If an individual had more than one inpatient admission that met our inclusion criteria, we selected the first admission as the index admission for all analyses.
Opioid misuse was determined by the presence of one or more ICD-9 diagnostic codes for opioid abuse, dependence, poisoning, or adverse effects (304.0x, 304.7x, 3.05.5x, 965.0x, E850.0–E850.2, E935.1, and E935.2), whether or not the code was for the primary diagnosis. We excluded inpatient admissions with diagnoses of suicide and self-inflicted poisoning (E950.0–E950.5) or assault by poisoning (E962.0).
Use of FDA-approved medication for the treatment of opioid dependence was defined as a prescription fill for buprenorphine or naltrexone or a paid claim for methadone. We identified use of buprenorphine and naltrexone for treatment of substance use disorders by using the National Drug Code (NDC) on the prescription drug claims. Federal regulations require that patients who are receiving methadone to treat opioid dependence receive their medications from a certified opioid treatment program; therefore, we identified methadone for treatment of substance use disorders by using Healthcare Common Procedure Coding System service codes. We determined whether a prescription for any of these three types of substance use disorder medications was filled within 30 days of the discharge date of the index admission.
We also defined four additional classes of prescription medication use in the 30-day postdischarge window: antidepressants, antipsychotics, benzodiazepines, and opioid pain medications, such as oxycodone methadone, morphine, fentanyl, hydrocodone, and tramadol. The use of methadone for pain was identified as a prescription fill. We included benzodiazepines, which are contraindicated in the population of individuals with an opioid-related hospitalization because of the increased risk of poor outcomes associated with the combined use of opioids and benzodiazepines (
11). We included antidepressants and antipsychotics because a preliminary analysis of the cohort in the online MarketScan Treatment Pathways tool showed that prescriptions for these medications were common among the cohort prior to hospitalization.
Results
We identified 36,719 patients with an inpatient admission for opioid abuse, dependence, or overdose that met our inclusion criteria; 42% (N=15,432) were women, and 58% (N=21,297) were between the ages of 18 and 35.
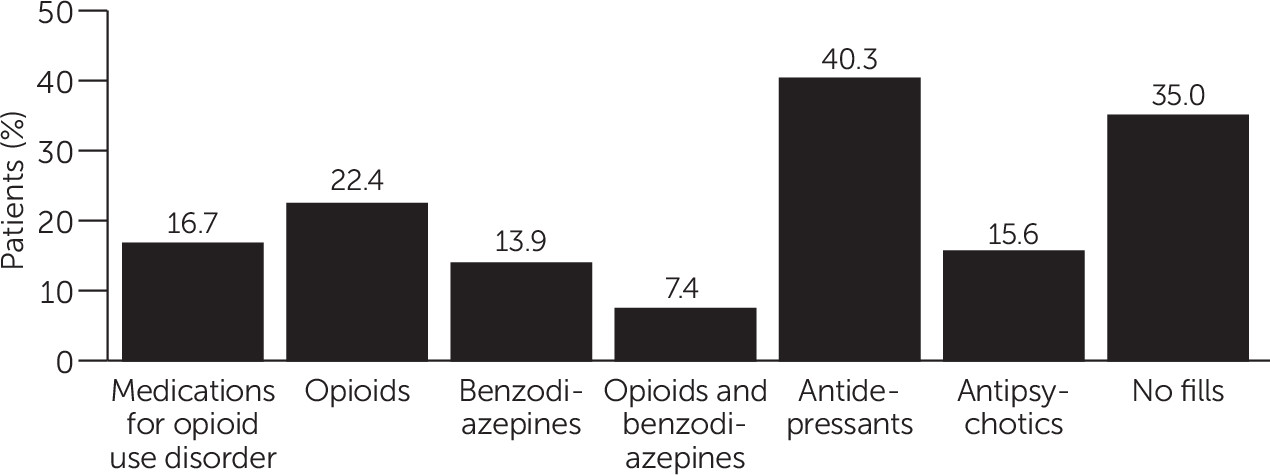
Figure 1 shows the frequency of prescriptions in the 30-day postdischarge window. The categories shown are not mutually exclusive. Less than a quarter (16.7%, N=6,132) of patients received any FDA-approved medication for opioid use disorder in the 30 days following discharge. The most commonly filled prescription was antidepressants, received by 40.3% (N=14,798) of patients in the sample. Antipsychotic prescriptions were filled by 15.6% (N=5,728) of patients, and 22.4% (N=8,225) filled a prescription for an opioid pain medication. Benzodiazepines were filled by 13.9% (N=5,104) of patients, making them the least frequently filled drug in the postdischarge period among the five classes examined. Additionally, 7.4% (N=2,717) of the sample filled prescriptions for both a benzodiazepine and an opioid pain medicine within 30 days of discharge. Thirty-five percent (N=12,852) of the sample did not have any prescription fills in the 30-day postdischarge window.
Discussion and Conclusions
Many studies have shown that FDA-approved opioid dependence medication is an important component of treatment services for opioid abuse or dependence. Recent research has also shown that 40% of privately insured individuals received no follow-up services within 30 days following an opioid-related hospitalization and that only 11% received the recommended combination of both medication and therapeutic services (
12). Given the high rates at which follow-up services are not provided and the clinical recommendations in support of use of medications, the relatively low rate of opioid dependence medication reported in this study is of concern. More research is needed to understand the policy, structural, and financial barriers facing patients with an opioid use disorder in trying to access outpatient services that include opioid dependence medication.
In addition, the postdischarge use of opioid pain medications and benzodiazepines observed in this study also raises concerns. Some prescribing of opioid pain medication following an opioid-related hospitalization may be due to the prescriber’s being unaware of the patient’s hospitalization. Prescription drug monitoring programs and notification of providers on the basis of insurance claims can help make prescribers aware of opioid-related hospitalizations, although patients can continue to obtain opioid pain medicine by changing providers, paying for prescription opioids with cash, or seeking opioids illegally (
13).
The combined use of benzodiazepines and opioid medications is associated with an increased risk of adverse outcomes, including fatal opioid overdose (
11,
13). The finding that 13.9% of patients filled a benzodiazepine prescription, 22.4% filled an opioid prescription, and 7% filled both after an opioid-related hospitalization suggests that targeted outreach to physicians and patients about recommended prescribing practices and the risks associated with the combined use of benzodiazepine and opioid use may be warranted.
A limitation of this study was that it measured prescriptions filled, not prescriptions written or medications taken. Patients may not fill prescriptions that providers prescribe. They may also not take the medication after the prescription has been filled. The correspondence between medications prescribed and medications taken by the patient has been shown to vary by drug (
14). Analysis of physician prescribing and patient consumption of medications requires data beyond what are typically available in insurance claims.
Another limitation of the study was that some individuals who were hospitalized for an opioid overdose may not have deliberately misused opioids. We could not distinguish between diagnoses for opioid overdose that were related to deliberate misuse or addiction and those from accidental misuse or complications of prescribed use.
Finally, it should be noted that this study assessed patterns of prescription fills in a commercially insured population; therefore, interpretations about patterns of prescription medication use in an uninsured population or in a population covered by Medicaid should be made with caution.
Initiation of buprenorphine and naloxone in the emergency department, along with screening, brief intervention, and referral to treatment, has been shown to improve postdischarge outcomes for patients seen in the emergency department for opioid dependence, including patient engagement in treatment for substance use disorders (
15). Further research will be needed to identify barriers to postdischarge treatment at the patient and provider levels and to determine strategies to address them.