Antipsychotic drugs are a common component of the therapeutic strategy for patients with serious mental illness (
www.rcpsych.ac.uk/mental-health/treatments-and-wellbeing/antipsychotics). Although U.K. and international guidelines recommend antipsychotic monotherapy (
1,
2), antipsychotic polypharmacy (hereafter, polypharmacy; defined as the concurrent use of two or more different antipsychotic agents) is common in clinical practice (
3,
4).
The most common rationale for polypharmacy is to improve therapeutic response when the response to monotherapy is considered inadequate (
1). However, there is little empirical evidence that polypharmacy has higher efficacy than monotherapy. A Cochrane systematic review (
5) of randomized controlled trials (RCTs) concluded that although polypharmacy might be superior to monotherapy in certain clinical situations, the evidence was too heterogeneous to derive firm conclusions. Significant risks associated with polypharmacy have been reported, particularly excessive dosing (
6), which can, in turn, result in adverse effects such as metabolic syndrome (
7), cognitive impairment, extrapyramidal side effects (
8), and cardiovascular disorders (
9). Polypharmacy efficacy and adverse effects contribute to changes in broader patient outcomes reflecting overall polypharmacy effectiveness. Whether polypharmacy is a valid therapeutic option or a “dirty little secret” (
10), it remains prevalent, and empirical evidence on its effectiveness is needed.
Our study followed a cohort of 17,255 patients with serious mental illness over time to make inferences about polypharmacy effectiveness in terms of three outcomes: unplanned hospital admissions, emergency department (ED) visits (accident and emergency [A&E] visits in the United Kingdom), and mortality. We constructed the antipsychotic prescribing profile of patients from primary care records that we linked to hospital and mortality data. The argument underpinning a cohort study design is that effectiveness is assessed under usual circumstances of health care practice rather than ideal RCT circumstances. As with all observational studies, validity relies on rigorous design and adjustment of confounding factors to minimize selection bias.
Although significant progress toward this direction has been made by two studies from Denmark (
11) and Finland (
12) that focused on the effect of polypharmacy on mortality, studies that explored associations between polypharmacy and inpatient hospitalizations (
13,
14) and ED visits (
15) had important weaknesses that stemmed from failure (or inability because of lack of data) to model the timing of polypharmacy episodes and outcomes. In the current study, we have improved on the fundamental issue of confoundedness by using a Cox survival analysis model that analyzes time to each outcome adjusting for both time invariant confounders and time-dependent polypharmacy and monotherapy (
16).
Methods
Data Sources
Our primary data source was the Clinical Practice Research Datalink (CPRD GOLD), which includes information on individual patients from family practice records, such as diagnoses, referrals, laboratory results, prescriptions, and immunizations. CPRD is sourced from participating U.K. general practices (GPs) that use the VISION software system and is broadly representative of the English population with respect to age and gender but not region.
CPRD records from English practices were linked to inpatient hospitalizations and A&E visits from Hospital Episode Statistics as well as mortality data from the Office of National Statistics. To preserve anonymity, the data linkages were carried out by the trusted third-party NHS Digital. Information was provided by CPRD for all patients who were eligible for linkage and had a diagnosis of serious mental illness.
Ethical Approval
The study protocol was approved by the Independent Scientific Advisory Committee (protocols 14-168 and 15-213).
Sample
The period from January 1, 2000, to March 31, 2014, was used for our sample. The observation period for each patient varied. The entry date to the sample was defined as the date on which the following conditions were met: patient had been diagnosed as having serious mental illness in primary care, patient was age 18 or older, patient was registered with a participating practice for at least 365 days, and patient was not hospitalized within the past 90 days. The latter two conditions were imposed to ensure that sufficient information on the patients’ medical history was available and because patients who were recently discharged from the hospital were at higher risk of readmission.
The observation period for each patient ended on March 31, 2014, or on the date of death or the end of registration with the practice, whichever came first. Patients were included in the sample if they had at least one antipsychotic drug record during the observation period. Because A&E data were available only beginning in year 2007–2008, the analysis of ED visits was limited to patients with an entry date after March 31, 2007.
Patient Outcomes
We investigated the association between polypharmacy and the occurrence of three outcomes: unplanned hospital admissions (all-cause), ED visits, and mortality.
Definition of Polypharmacy
There is no consistent definition of polypharmacy in the literature. We defined polypharmacy as the concurrent use of two or more antipsychotic substances for at least 30 days. The overlap period allows for cross-tapering between substances. A longer overlap period has a higher risk of misclassifying polypharmacy as monotherapy, whereas a shorter overlap may misclassify switching between substances as polypharmacy. We therefore explored overlap periods of 14, 60, and 90 days as sensitivity analyses. We considered 33 antipsychotic substances covering first-generation antipsychotics, second-generation antipsychotics, and depot antipsychotics (
17,
18) [see
online supplement].
CPRD data provide the date a prescription was issued, but the duration of prescriptions is poorly recorded. Therefore, we inferred treatment duration from the total quantity (number of units) prescribed and the numeric daily dose (number of units per day). The latter was missing for 23% of prescriptions. For these prescriptions, we imputed the numeric daily dose by using an imputation strategy [see online supplement]. Less than 0.02% of prescription records were dropped from the analysis because they had implausibly large estimated duration. From the prescription dates and durations, we constructed the patient’s medication profile: times at which the patient was on any antipsychotic medication and on polypharmacy.
We calculated two measures of polypharmacy prevalence. First, the annual prevalence of polypharmacy was defined as the number of patients with at least one polypharmacy episode in a year divided by the total number of patients observed during that year. Second, the rate of polypharmacy was defined as the sum of all patients’ polypharmacy days in a year over the sum of all patients’ days at risk of polypharmacy in that year. The latter measure is an improvement over the commonly reported point estimates of polypharmacy prevalence, which measure the proportion of eligible individuals on polypharmacy on a given day [see online supplement for a proof of the method’s accuracy].
Covariates
We used Read codes (the diagnostic codes used in U.K. primary care) recorded over the entire patient’s history and our own clinical expertise to define three diagnostic categories: schizophrenia and other psychoses, bipolar disorder and affective psychoses, and a diagnosis from each group [see online supplement for codes].
All other covariates were measured at the date of entry to the study sample. We controlled for age, gender, age-gender interactions, the number of comorbid conditions as defined by the Charlson Comorbidity Index (
19), a diagnosis of depression, alcohol consumption and smoking status, the number of GP contacts (face-to-face visits and telephone calls) in the last year, and the deprivation level associated with the patient’s area of residence as captured by the English Index of Multiple Deprivation. We approximated ability to access secondary care by the distance from the patient’s GP to the nearest psychiatric inpatient hospital and general hospital and by whether the practice was located in a rural area. Finally, we controlled for the year in which the patient entered the sample and the time since first diagnosis [see
online supplement for details on the explanatory variables].
Statistical Analysis
Semiparametric Cox hazard models (
20) were applied to estimate the effect of polypharmacy on the time to the first occurrence of each of the three outcomes. The model adjusts for censoring, which may occur because a patient dies, registration with the practice ends, or the study period ends. The follow-up period (time from entry to the sample until the outcome occurs or censoring) is different from the observation period for outcomes other than death.
An individual may have multiple polypharmacy episodes. On each day during the study period, the patient was in one of three states: received no antipsychotic medication, monotherapy (used one antipsychotic or more than one but for less than 30 days), and polypharmacy. To model this approach, we used two time-varying binary variables: “no antipsychotic substance,” which takes a value of 1 during periods when the patient is not on an antipsychotic drug and 0 otherwise, and “polypharmacy,” which takes a value of 1 during periods that the patient is on two or more antipsychotic substances for more than 30 days and 0 otherwise. The results are interpreted with regard to monotherapy, which was the reference category.
All coefficient estimates are reported as hazard ratios (HRs), in which an HR of greater than 1 indicates an increase in the risk of the outcome associated with a unit change in the explanatory variable, and vice versa for an HR of less than 1 [see online supplement for details on the survival analysis]. All analyses were performed in Stata, version 14.
Results
All patients were prescribed an antipsychotic substance at some point during the observation period.
Table 1 provides descriptive statistics. Unplanned admissions and mortality outcomes were studied by using the same sample of 17,255 patients from 215 practices. These patients were observed for 5.7 years on average, and 12.9% had at least one polypharmacy episode during the observation period. The average number of polypharmacy episodes per patient on polypharmacy was 5.5, and the mean polypharmacy episode length was 66 days (range 2–2,340).
For the unplanned admissions analysis, the average follow-up period was shorter than the observation period (3.6 years), with 8.8% of patients having at least one polypharmacy episode during this period. Almost 52% (N=8,916) of the patients had an unplanned admission, and of those patients, 7.9% had at least one polypharmacy episode before the admission.
For the mortality analysis, the average time to death or censoring was 5.7 years. Of the 604 patients who died (3.5%), 8.6% had received polypharmacy.
The sample for ED visits covered a shorter period (April 1, 2007, to March 31, 2014), totaling 13,247 patients from 215 practices. Of the 7,523 patients with an ED visit, 6.8% had received polypharmacy.
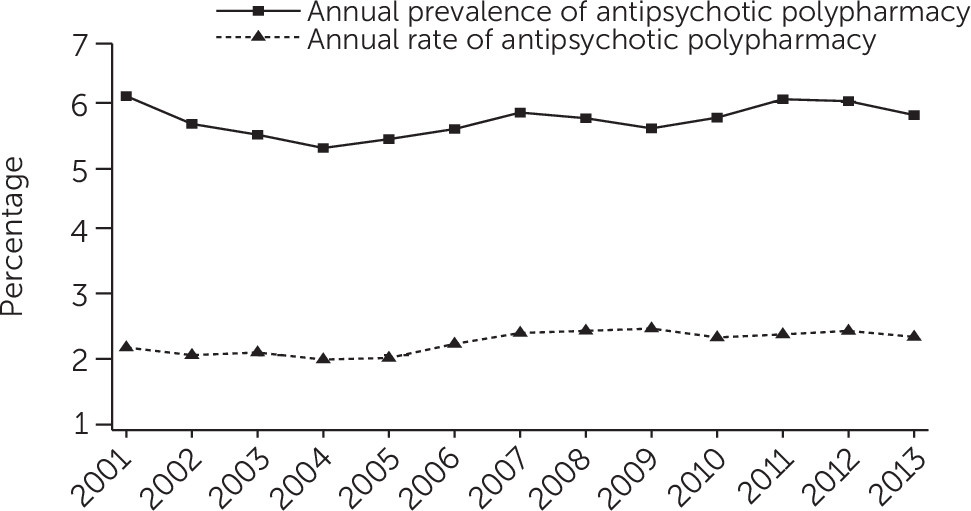
Figure 1 shows that annual prevalence of polypharmacy fluctuated between 5% and 6%, whereas the polypharmacy rate was around 2%. Polypharmacy rate estimates are lower than the annual polypharmacy prevalence because the former reflects both whether a patient is on polypharmacy during the year and the total duration of polypharmacy episodes [see
online supplement for figures of the annual prevalence of polypharmacy and the polypharmacy rate for different overlap periods].
Summary statistics for the explanatory variables are presented in
Table 2. About 35% of patients had at least one of the Charlson index morbidities, and 13% were diagnosed as having both schizophrenia and bipolar during the observation period.
Table 3 presents the survival analysis estimates for the two time-varying variables for our main specification assuming an overlap period of 30 days. Being on polypharmacy (relative to monotherapy) was not statistically significantly associated with the risk of unplanned admission, death, or ED visit. Not being prescribed any antipsychotic substance increased the hazard (relative to monotherapy) of an unplanned admission to hospital by 8.2% (95% CI [confidence interval]=3.0%–13.6%) and the hazard of an ED visit by 18.6% (95% CI=13.5%–23.9%), but it had no effect on mortality risk [see
online supplement for estimates of the other explanatory variables]. Having a diagnosis of both schizophrenia and bipolar disorder increased the hazard of an unplanned admission by 20% (HR=1.20; 95% CI=1.12–1.29).
Table 4 shows the results of sensitivity analyses that explored the impact of changing the length of overlap in the definition of polypharmacy. The estimated relationships were generally insensitive to the length of overlap. The only exception was unplanned admissions: when the lower boundary of the overlap duration was reduced to 14 days, polypharmacy was associated with an increased hazard of unplanned admission by about 21% compared with monotherapy.
We also estimated our survival models for psychiatric hospitalizations, which were a subset of all unplanned admissions. The results showed no association between psychiatric hospitalizations and polypharmacy [see online online supplement].
Discussion
This study is a step forward toward understanding the links between polypharmacy and health care utilization and mortality. As with all observational studies, validity relies on rigorous design and adjustment of confounding factors to minimize selection bias. We addressed this fundamental issue by using a three-step strategy.
First, we constructed the antipsychotic prescribing profile of patients from primary care records. In the United Kingdom, family practices provide the majority of care for patients with serious mental illness (
21), including the management of long-term prescriptions. Therefore, unlike previous studies that used solely hospital data to investigate polypharmacy (
22), we defined polypharmacy and monotherapy from primary care data. Second, we linked primary care data with hospital and mortality data at the patient level to determine the sequence of polypharmacy episodes and hospital utilization and mortality. Third, we used a Cox survival analysis model that analyzed time to each outcome by adjusting for both time-invariant confounders and time-dependent polypharmacy and monotherapy (
16). By specifying polypharmacy as a time-dependent variable, we addressed the statistical challenge arising in cases in which the exposure is not present throughout the entire time of observation.
The use of a large linked data set coupled with a suitable survival analysis model provided more robust estimates of the effects of polypharmacy on outcomes than would be possible with aggregate data or a cross-sectional design. We found that the annual polypharmacy prevalence fluctuated over time between 5% and 6%. This figure is not comparable with other studies because of diversity in the definition of polypharmacy and differences in the sample characteristics and methodology. A large international study estimated a global median of 20% (
23), but there was considerable variation between and within geographic locations (
23,
24).
Higher rates of polypharmacy have been estimated for the United Kingdom, but the patients included in those studies were prescribed at least one antipsychotic at the date of data collection; moreover, polypharmacy was defined as the concurrent use of more than one antipsychotic on that single date (
25,
26), a definition that is likely to overestimate polypharmacy. Kadra et al. (
22), using a more comparable approach and a 6-week overlap, found a polypharmacy rate of 11.5%. The lower estimate of polypharmacy prevalence in our study may be because patients can be at risk of polypharmacy for a fraction of a calendar year, whereas in Kadra et al.’s study, patients were followed for an entire 6-month period.
Current U.K. guidance (
1) recommends antipsychotic monotherapy as a treatment option, and our results provide further supportive evidence establishing a negative association between antipsychotic monotherapy and hospitalization. This finding may be due to the fact that drug therapy helps to stabilize patients’ conditions and allows better management of their general health. Being prescribed an antipsychotic may be associated with closer or more regular clinical monitoring in the primary care setting, as set out in the guidelines that recommend that prescription of an antipsychotic should be considered as “an explicit individual therapeutic trial” (
8), accompanied by detailed requirements for monitoring. The latter may facilitate timely diagnosis and treatment of health problems, avoiding the need for hospital care.
It is widely believed that polypharmacy increases mortality and hospitalizations, but there is a lack of methodologically sound studies to support this assumption. To our knowledge, the only previous study that used nationwide data for medication prescriptions and appropriate methods to adjust for confounding factors was conducted by Tiihonen et al. (
12). They investigated the impact of polypharmacy on mortality by using a cohort of 2,588 patients from Finnish hospital data and concluded that polypharmacy is not associated with increased mortality. This conclusion is reinforced by this study, which used a significantly larger cohort of 17,255 patients with a serious mental illness diagnosis in primary care. Our study further concludes that there was no association between polypharmacy and inpatient hospitalizations or ED visits, contrasting the positive correlations found in previous studies (
13–
15).
The finding that polypharmacy was not significantly associated with any of the three outcomes suggests that the effectiveness of polypharmacy and monotherapy is comparable. When we used a shorter overlap period (14 days or longer) that captured more cross-tapering in the definition of polypharmacy, we observed an increase in the risk of unplanned admission. One explanation is that patients who change drugs might have more unstable disease profiles or that changing drugs further destabilizes their condition. This finding suggests a need for close monitoring in the first few weeks of cross-tapering, when the risk of unplanned hospitalization is higher.
U.K. guidelines (
1) recommend against combining antipsychotic drugs except as a last resort. These recommendations are based on limited supportive evidence for superior efficacy of polypharmacy over monotherapy as well as concerns that combined antipsychotics are associated with an increased risk of side effects. Our study cannot draw conclusions on the polypharmacy effect in terms of efficacy and tolerability; furthermore, despite its advanced design, it cannot completely overcome the limitations of observational studies and therefore cannot substitute for RCTs.
This study’s contribution lies in providing real-world evidence on the effectiveness of polypharmacy. There are three main limitations to the study. First, the measures of health status and health care utilization before diagnosis of serious mental illness may not fully depict the complexities of health status, including severity of the condition. Second, imputing the treatment duration for a number of prescriptions may introduce measurement error in the calculation of polypharmacy. Last, we have explored the effect of polypharmacy on broadly defined outcomes. Future research could investigate whether effects vary by reason for admission or for particular combinations of antipsychotic medication.
Conclusions
Our study examined the overall effectiveness of polypharmacy relative to monotherapy by investigating associations between polypharmacy and three patient outcomes. We found no evidence of a positive or negative effect of polypharmacy on mortality, inpatient hospitalizations, and ED visits. At a policy level, these findings do not rule out polypharmacy options but highlight the need for further research on the appropriateness of polypharmacy.