Although consistent antipsychotic medications are recommended treatment for individuals with schizophrenia (
1,
2), at least half do not take oral medication as prescribed, leading to relapse and high health care costs (
3–
5). Poor antipsychotic adherence is estimated to cost more than $21 billion annually in the United States (
6).
Treatment guidelines recommend long-acting injectable (LAI) antipsychotic medications for patients who have trouble taking daily oral medication (
7). Use of LAI antipsychotics improves medication consistency and is associated with lower rates of rehospitalization (
8), reduced costs (
9), and lower mortality (
10). LAI antipsychotics can help disentangle lack of medication efficacy from the effects of poor adherence and identify the onset of problems with adherence (
11–
13).
Despite multiple benefits, LAI medication is underutilized (
11,
13). For providers, key reasons include an inability to identify problems with adherence, misconceptions about and lack of familiarity with LAI antipsychotics, and fear that recommending LAI medication may appear coercive and harm therapeutic alliance (
11,
14–
16). Providers lack confidence in their LAI prescription recommendation and do not know how to make offers of LAI medication that are acceptable to patients (
17). Consumers are often unaware that LAI antipsychotics are an option (
18,
19), have misconceptions and concerns about side effects, and may confuse LAI medication with short-acting injections given in emergency situations (
11,
14,
16).
Although guidelines and position papers highlight the need to change prescriber attitudes and increase LAI medication use, few studies have been designed to increase clinic-wide use (
7,
11,
12,
14). In a randomized controlled trial, a structured approach using goal setting, action planning, initiating treatment, and nurturing motivation (GAIN) to increase LAI medication use was accepted by consumers but did not increase LAI medication use compared with clinician offers as usual (
20). A case vignette study suggested that credible evidence of nonadherence may make providers more likely to prescribe LAI antipsychotics (
21).
We developed the Multilevel Facilitation of Long-Acting Antipsychotic Medication Program (MAP) to address the barriers leading to LAI medication underutilization and initiated a two-state, four-site pilot program examining feasibility, acceptability, and preliminary impact on LAI medication use. MAP contains three components: the not receiving optimum benefit (NOB) checklist (see the online supplement to this article) to identify individuals for whom a change to LAI antipsychotics might be beneficial; a patient-facing, shared–decision-making (SDM) video; and academic detailing (AD) targeting prescribers to improve their understanding of LAI antipsychotics and how to make successful LAI prescription offers. In this article, we describe the challenges to using various components of MAP during this preliminary feasibility initiative.
Methods
Participants included community mental health clinics within the same health systems in Texas (November 2017–March 2019) and Ohio (April 2018–March 2019). One site in each state was randomly designated as an NOB-only clinic and the other as a MAP clinic. The project was determined exempt by the institutional review boards of the principal investigator’s universities and was approved by the research oversite committees of participating clinics.
MAP Treatment Targets and Components
MAP is a multicomponent intervention involving multiple stakeholders (administrators, clinicians, patients) in the processes of increasing the appropriate use of LAI antipsychotics within an SDM framework. The program was designed to address barriers to LAI medication use (see online supplement). MAP focuses on getting buy-in from clinic administrators in a brief AD session provided by a key opinion leader (M.S.). The program provides information on the scope and consequences of problem adherence and the role of LAI antipsychotics in the treatment of individuals not receiving optimal benefit from current treatment as well as access to tools designed to help improve clinic efficiency and quality of care with LAI antipsychotics. After administrators determine that increasing the appropriate use of LAI medication is a desired goal, they identify a “change champion” in the organization who will work with staff and prescribers to use MAP tools, address barriers, repeat essential messages, and provide feedback on prescribing practices. MAP consists of three components, which are presented next.
NOB only.
The NOB checklist allows prescribers to identify individuals not receiving optimal benefit from their current antipsychotic treatment. The checklist does not rely on a prescriber identifying poor adherence. It is composed of five yes-or-no questions to identify individuals for whom a possible change to LAI antipsychotics should be considered (see online supplement) (
7,
12). The provider also records whether LAI medication was offered and whether it was accepted. Prescribers were asked to complete an NOB checklist for all patients with schizophrenia or schizoaffective disorders taking oral antipsychotics. Providers in NOB-only clinics received 10 minutes of in-service training on LAI medication, were trained on using the NOB checklist, and were asked to complete at least one checklist during the clinic visit for all patients receiving oral antipsychotics.
AD.
In addition to using the NOB checklist, MAP included AD that targeted clinicians. AD sessions occurred quarterly at MAP clinics. Five web-based AD videos, which were 5–7 minutes in length and unbranded, described LAI medication benefits, side effects, and dosing to increase comfort with prescribing LAI antipsychotics. Scientific content and balance were evaluated by an advisory group of six prescribers from clinics and hospitals in Texas not affiliated with the research. AD videos illustrate how to make offers in an SDM dialogue that tie LAI antipsychotics to a person’s recovery goals and that are acceptable to consumers (
17). In the AD videos, the latest research findings are discussed to increase confidence in prescribing LAI antipsychotics (
11,
13–
17) and to dispel common misconceptions about the extent of coercion and stigma attached to LAI medication—attitudes that research indicates are more commonly held by practitioners than by patients (
16).
AD is highly effective in changing clinician behavior. It begins with understanding the clinician’s baseline knowledge and practices, limits content to a “digestible” key message, and provides a memorable summary (
22). Clinic providers are given the option of watching the entire series in a 35-minute block during a provider meeting or watching individual segments at separate times. AD videos (
https://www.youtube.com/watch?v=RP2JpaY0BvU&feature=youtu.be) are followed by a short consultation with an expert (in this study, M.S.) or a local champion.
SDM.
SDM is used to target individuals in treatment. Consumers receiving oral antipsychotics are approached by a peer specialist and asked to watch an SDM video (
https://youtu.be/nl-g-Zhwg0Q) teaching a simple acronym called TAC-Review (tell, ask, choose, review) to help individuals communicate their values and preferences to the prescriber and to get the most out of visits (
23). In our study, a half-time peer specialist was asked to be available to show the SDM video. The video highlights individual goal setting, promotes personal decision making, presents medication as a recovery tool, and destigmatizes LAI antipsychotics. Pros and cons of LAI medication versus tablets are presented by multiple individuals from a variety of demographic backgrounds. The peer answers questions about his or her own experience of LAI antipsychotics and provides a written decision tool for the consumer (see online supplement) (
24). Clinic staff identify eligible individuals before clinic check-in and alert the peer when these individuals arrive. Ideally, the peer approaches the individual before the visit with the prescriber. Clinic space and availability of peers dictate the number of individuals who can be approached. Over time, in our study, most individuals could be offered an opportunity to view the video.
Outcomes
Feasibility, barriers, and facilitators.
Feasibility issues, barriers, and facilitators were recorded on weekly problem-solving calls and visits with staff. Quarterly quantitative assessments of barriers and facilitators developed by the authors were also completed. Attempts to address issues were made in real time.
MAP use.
In all clinics, we examined use of MAP components by calculating the number of individuals for whom an NOB checklist was completed, divided by all eligible individuals (i.e., those with schizophrenia spectrum disorders on oral antipsychotics). For MAP clinics, we examined the number of individuals viewing the SDM video divided by the number of eligible individuals.
NOB checklist.
We assessed the preliminary validity of the NOB checklist by examining relationships between NOB checklist scores and LAI medication offers and acceptance.
Exploratory efficacy.
Administrative data provided by the clinics indicated the number of individuals with schizophrenia spectrum disorders who were prescribed oral antipsychotics versus LAI antipsychotics in specific months.
Data Analysis
Barriers and facilitators were recorded from calls and quarterly assessments. Descriptive statistics quantified use of MAP components. We examined first-time NOB checklists completed across clinics to see whether there was an association between any “yes” responses on the NOB checklist (zero versus 1+) and whether LAI medication was offered. We also assessed the association between number of NOB checklist “yes” responses (one to five) and LAI medication offers. In the subsample offered LAI antipsychotics, we examined whether an association existed between NOB checklist “yes” responses (yes or no and specific score) and LAI medication acceptance. Because NOB checklist scores represent ordinal data, we tested associations using Mantel-Haenszel chi-square tests, 2×2 and 5×2, respectively (
25).
Regarding route of medication, data from Texas represented monthly prescriptions in 14 consecutive months ranging from January 2018 to February 2019, with January to April being baseline months before program initiation. Data from Ohio represented prescriptions from 6 irregularly spaced months spanning 2 years: February and November 2017, February and April 2018, as well as February and March 2019, with February 2017 representing the baseline. Counts were converted to proportions of LAI antipsychotics, and data were analyzed by state.
Statistical analyses for this exploratory outcome used the Mann-Kendall S statistic, a count of the number of times later observations are higher on the variable of interest than earlier data minus the number of times the later data are lower. Mann-Kendall tests were used to examine the trends for MAP and NOB clinics in both states. We tested differences between MAP and NOB clinic trends using the differences-in-differences method, in which the NOB clinic LAI medication proportion is subtracted from the MAP proportion in each time period and the Mann-Kendall test is applied to this series of differences.
Results
Program Feasibility, Barriers, and Facilitators
One administrator in each state responsible for both state clinics assisted with rollout. Procedures differed by state. In Texas, MAP was facilitated by regular onsite check-ins with administration and providers as well as by the integration of MAP components into clinic workflow. A staff member identified eligible individuals attending clinic visits each day, and NOB checklist prompts were added to a procedures list for their visit. Barriers described on calls and questionnaires included days with no staff available to insert NOB checklist prompts and providers reporting time constraints for completing NOB checklists. In-person collection of completed NOB checklists each week by administrative staff was reported to increase the weekly number of NOB checklists collected.
Providers reported that the checklist helped identify individuals who may benefit from LAI antipsychotics. Conducting AD sessions at regularly scheduled staff meetings was reported to facilitate provider attendance, and providers at both MAP sites elected to view all the videos at one time each quarter. Make-up sessions were conducted as needed. One part-time peer showing the SDM video limited the number of individuals who could be approached. Spontaneous comments from patients following the video indicated that that they enjoyed the video, and many stated that they would discuss LAI antipsychotics with their doctor. Some reported disappointment that their medication was not available in injectable form.
In Ohio, less assistance was available, and sweeping administrative changes, including departure of the local champion and medical director, affected the use of MAP. AD was conducted at regular staff meetings, and slide handouts were provided for providers unable to attend. In some cases, a pharmacist completed the NOB checklist and communicated results to the prescriber. There was difficulty developing a process to integrate NOB checklist completion into clinic workflow. Without a local champion, provider buy-in to use the NOB checklist was limited. Clinics were not familiar with the use of peer specialists and did not use the SDM video. Thus, Ohio clinics were NOB only and NOB+AD and had difficulty using MAP.
Intervention Use
A total of 22 providers participated: five and seven providers (N=12) for MAP clinics and four and six providers (N=10) for NOB-only clinics in Texas and Ohio, respectively. Two part-time telemedicine providers in Texas did not participate. All providers received initial NOB-only or AD training, and MAP providers participated in at least two of the three quarterly follow-up sessions. Of the providers, nine of 12 (75%) attended all three sessions.
In Texas, NOB checklists were completed for 379 of 750 eligible patients (51%). The peer approached 200 individuals and showed the video to 178 of 750 patients (24%). In Ohio, the NOB checklist was completed on 95 of 617 individuals (15%), and the video was not shown.
NOB Checklist
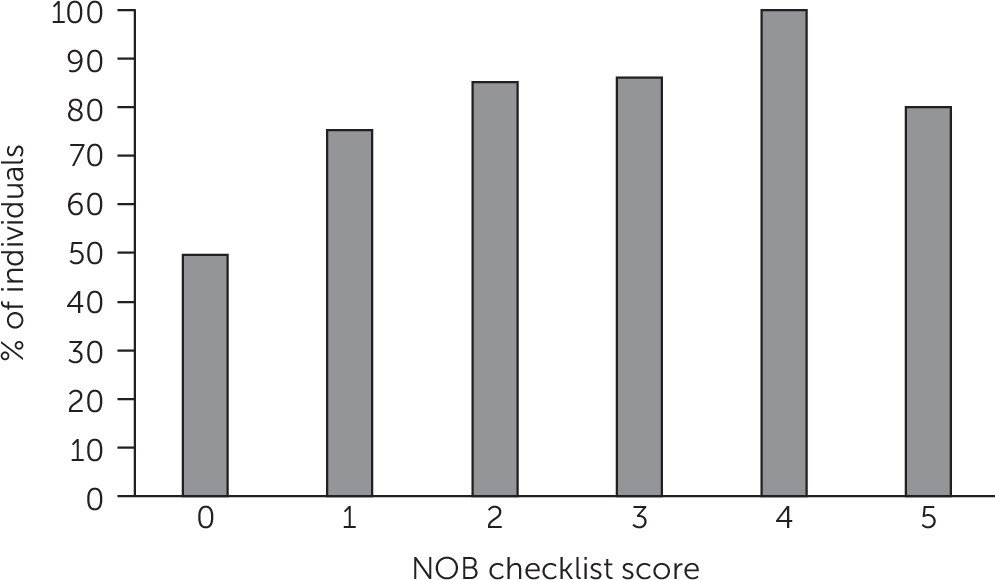
A total of 474 initial NOB checklists were obtained across sites. Six were incomplete, leaving 468 for analysis. Of the consumers, 201 of 468 (43%) received an NOB checklist score of ≥1, and 299 of 468 (64%) were offered LAI antipsychotics.
Any “yes” response on the NOB checklist was associated with LAI medication offers as reported by the provider. Of the patients with a score of zero, 134 of 267 (50%) were offered LAI antipsychotics, compared with 166 of 201 patients (83%) with a score greater than zero (χ
2Mantel-Haenszel [MH]=53.28, df=1, p<0.001). A higher number of “yes” responses was related to increased likelihood of an offer (χ
2MH=47.59, df=5, p<0.001) (
Figure 1).
A significant association was found between NOB checklist scores (zero vs. one “yes” response) and LAI medication acceptance (χ2MH=27.02, df=1, p<0.001). Of individuals with NOB checklist scores of zero, 18 of 133 (14%) accepted the offer, whereas 68 of 166 (41%) of those with at least one question scored as “yes” accepted the LAI medication offer. A significant association was found between number of “yes” responses on the NOB checklist and LAI medication acceptance (χ2MH=34.46, df=5, p<0.001) (see online supplement).
Use of LAI Antipsychotics by State: Preliminary Outcome
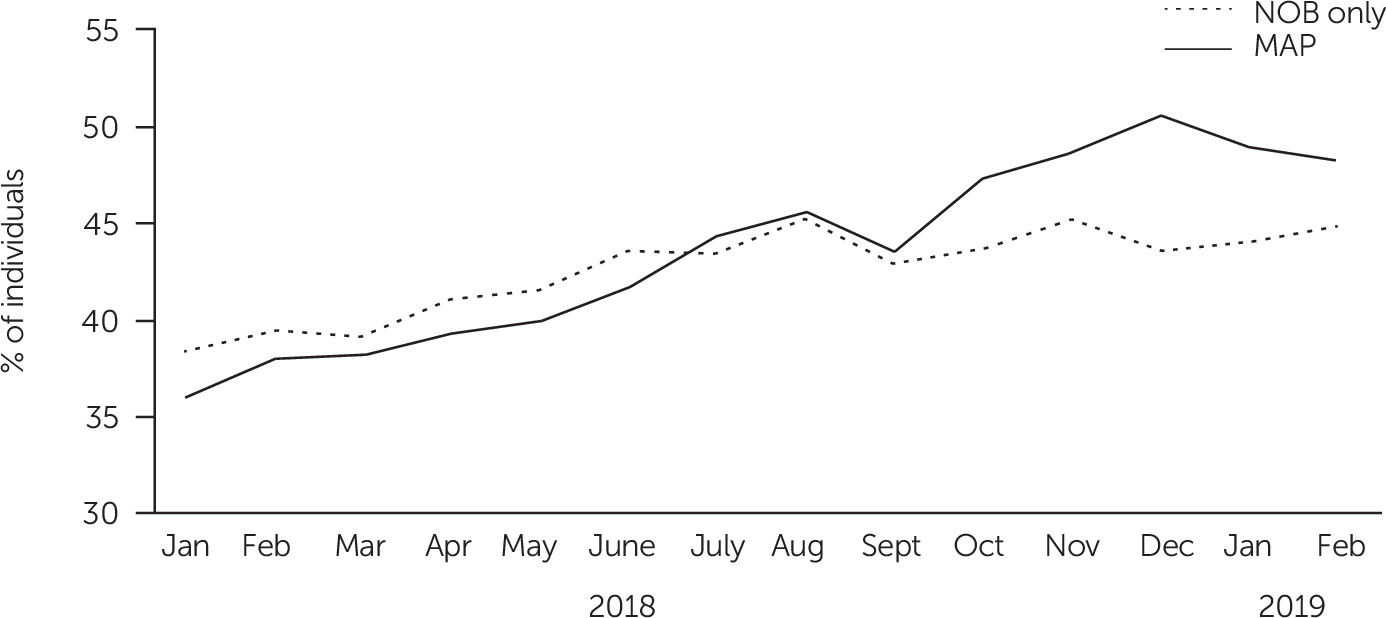
Figure 2 presents monthly LAI medication use in Texas and results of tests for linear trend. Findings suggest that the difference between MAP and NOB clinics systematically increased over time, with the proportion of LAI medication in MAP rising nearly twice as fast as in NOB clinics (Mann-Kendall p<0.01). In contrast, in Ohio, LAI medication use for the AD clinic (randomly assigned to MAP but unable to deliver the SDM component) increased slightly less than for the NOB clinic (Mann-Kendall p<0.55) (see online supplement).
Discussion and Conclusions
Poor adherence among patients with schizophrenia is associated with relapse and high costs (
3,
5). Emphasizing the potential benefits of LAI antipsychotics among both prescribers and consumers using MAP has the potential to increase discussion of LAI medication during visits. Because clinicians are not able to identify problem adherence reliably (
7), the NOB checklist may provide an alternate way to consider who may benefit. Research has indicated that prescribers may be uncomfortable making LAI prescription offers and may offer medication in ways that get a predictably negative response (
17). The AD component of MAP specifically addresses skills for presenting LAI antipsychotics to consumers. Finally, by empowering the consumer, an offer of LAI medication is made to someone informed of the pros and cons before a discussion with the provider, an approach long advocated in SDM (
26).
Some barriers to MAP rollout occurred. In Ohio, the NOB checklist was not integrated into clinic workflow. This outcome was likely a function of sweeping administrative changes, loss of a local champion, and limited prescriber buy-in. The NOB checklist was completed for half of all eligible people in the Texas clinics, with prescribers mentioning time constraints as an issue. Results suggest that feasibility may be improved by having administrative support and a local champion and by applying strategies that fit the use of MAP into clinic workflow. With the SDM video, peers were an important part of the treatment teams in Texas but were absent in Ohio. Alternative ways to implement SDM could include using the video as part of reimbursable medication education or skill building or showing it in a waiting area.
LAI medication use increased over time in all clinics, with nearly two-thirds of individuals with schizophrenia or schizoaffective disorder who received an NOB checklist being offered LAI antipsychotics. In addition, NOB checklist scores indicating suboptimal treatment response were associated with increased LAI prescription offers and acceptance. In Texas, where the full MAP was implemented, the MAP clinic significantly increased use of LAI antipsychotics compared with the NOB clinic.
The finding that NOB checklist scores were associated with LAI prescription offers and acceptance provides preliminary evidence that the NOB checklist may be useful. NOB checklist questions may sensitize clinicians and patients to the need for change. The NOB checklist may give providers a way of discussing change options and LAI medication recommendations confidently. With respect to the NOB checklist, if there were multiple reasons to look at a switch, clinicians, on telephone calls and in exit interviews, reported having more confidence in their LAI prescription offer. The NOB checklist may have sensitized those in treatment to consider a change. Although further study is needed to determine whether the NOB checklist and MAP might increase the appropriate use of LAI antipsychotics in community mental health clinics, encouraging both patients and providers to address medication route may empower both members of the dyad to select the option that fits best.
Our results suggest that the fully implemented MAP, including the patient as a key stakeholder, may offer an incremental advantage but may be challenging to implement. For resource-limited settings, the NOB checklist alone might be useful to increase LAI medication use, but this procedure needs further study. LAI medication increase over time despite limited NOB checklist use in Ohio may suggest that frequent administrative reminders to complete the NOB checklist and attendance at AD led providers to consider LAI antipsychotics more broadly or that prescribing was already moving in this direction. Although the findings of this report are preliminary, they have potential clinical implications. If feasible and effective, the NOB checklist, AD, and SDM could be modified to target other medications that are underutilized, such as clozapine.
Limitations of this study included that it was a preliminary feasibility trial in sites chosen by convenience. It included only four clinics and no control comparator. Increasing use of LAI antipsychotics may have been related to factors other than the NOB checklist and MAP, such as novel LAI antipsychotics approved by the U.S. Food and Drug Administration becoming available, clients on oral medication differentially dropping out of treatment, or spontaneous changes in prescribing. The NOB checklist was based on published literature and needs additional validation. Clinics were overburdened, likely contributing to limited NOB checklist use and irregular data collection. Because individual patient data were not collected, we were unable to examine types of individuals offered or accepting LAI medication. No data on reasons for not offering LAI antipsychotics were collected.
Despite these limitations, preliminary evidence suggests that MAP might be feasible in certain clinics and that it might increase appropriate use of LAI medication. Use of the NOB checklist and MAP need customization to overcome barriers to use and additional study to examine their potential impact on LAI medication use.