Hazardous drinking, that is, alcohol consumption that places individuals at risk for adverse health events, is a major public health challenge, particularly in low- and middle-income countries (LMICs) (
1–
3). Alcohol misuse is avoidable, yet results in high morbidity and mortality rates and ranks as the third-leading risk factor for poor health globally (
1–
4). Harmful alcohol use contributes to >200 types of diseases and injuries. Alcohol’s impact is worst among people in poor populations and in LMICs where disease burden per liter of alcohol consumed is greater than among individuals in wealthy populations (
2,
5,
6).
Mozambique is a low-income country where half of the population lives below the poverty line as set by the World Bank and disease burden is high. Although research on alcohol use in Mozambique is scarce, recent studies have revealed that binge drinking (episodes of excessive drinking, i.e., four or more drinks per episode for women and five or more drinks per episode for men) is frequent among drinkers in Mozambique. Two recent studies with Mozambicans ages 25–64 years found that 24%−29% of women and 49%−58% of men were current drinkers (having consumed at least one drink in the past year) (
7,
8). Approximately 60% of current drinkers consumed alcohol at least once or twice a week, and >40% reported binge drinking in the previous week. Three-quarters of drinkers reported consuming home-distilled traditional alcohol beverages with high alcohol content, which was strongly associated with binge drinking. Having depression and experiencing the death of a child were associated with hazardous drinking (a score of >4 on the Alcohol Use Disorders Identification Test [AUDIT]) (
9).
Mozambique’s HIV prevalence has increased by 15% since 2009 (
10). Alcohol is implicated in behaviors that increase the risk for contracting HIV and in poor HIV care adherence (
11). HIV is the second largest category of alcohol-attributable disability-adjusted life years (DALYs); alcohol-attributable disease burden worsens if the impact of alcohol consumption on HIV incidence and course is considered, with alcohol being responsible for 6.4% of all deaths and 4.7% of all DALYs lost in the African region (
12). Thus, decreasing hazardous drinking could improve HIV care outcomes.
The World Health Organization’s Mental Health Gap Action Programme (mhGAP) guidelines (
13,
14) recommend using the Screening, Brief Intervention, and Referral to Treatment (SBIRT) (
15) approach to reduce hazardous drinking. SBIRT is typically integrated into medical care to address substance use disorders (
16). However, there are challenges to implementing SBIRT with fidelity, obtaining the effectiveness observed in trials, and sustaining SBIRT (
17), particularly in LMICs (
18). Hazardous drinking services (HDS) in Mozambique are delivered in specialty clinics by psychiatric technicians trained in SBIRT and other HDS informed by mhGAP evidence. With one specialty clinic per district, each with 50,000–150,000 inhabitants, most individuals who engage in hazardous drinking are not served. Our study aims to address this challenge through work with the Mozambican Ministry of Health to task-shift SBIRT to community health workers (CHWs). We will build on the Ministry of Health’s mhGAP-Epilepsy Program (
19) and our community mental health trial, funded recently by the National Institute of Mental Health, which links study data to Mozambique’s electronic medical record system (
e-saúde) to track the impact of two SBIRT approaches on comorbid conditions, including HIV and tuberculosis (TB).
Mobile health technologies (mHealth) (
20), such as the mobile SBIRT (mSBIRT) application (
21), are a promising tool for widespread, cost-effective, and sustainable health service delivery in LMICs (
20,
22–
24). The mSBIRT application was designed for use by health care providers. It assists providers in quickly assessing a patient’s alcohol use risk level and guides providers through a brief intervention that is tailored to the patient’s responses. For LMICs such as Mozambique, identifying the most appropriate strategy to implement SBIRT in community HDS is crucial. Capitalizing on Mozambique’s task-shifting strategies and commitment to inform HDS scale-up with local treatment guidelines (i.e., SBIRT and mhGAP), this study will scale up SBIRT in the community. We will compare mSBIRT with the SBIRT–Conventional Training and Supervision (SBIRT-CTS) strategy to determine the most cost-effective approach for expansion and for overcoming implementation barriers.
Community Implementation of SBIRT using Technology for Alcohol use Reduction in Mozambique (Community I-STAR Mozambique) comprises a 2-year, cluster-randomized, hybrid implementation-effectiveness type-2 trial in 12 districts evaluated by mixed-methods analyses (
Table 1). Our first two aims will be accomplished throughout the trial. Aim 1 is to conduct an implementation impact evaluation by using the Reach, Effectiveness, Adoption, Implementation, Maintenance (RE-AIM) model and compare the adapted mSBIRT with SBIRT-CTS in terms of reach (primary outcome), adoption, implementation fidelity, and maintenance over time. Aim 2 is to compare clinical effectiveness and cost-effectiveness of mSBIRT and SBIRT-CTS overall and by patient’s gender, age, and urbanicity (
25). Aim 3 will be accomplished throughout the trial and the subsequent scale-up phase (i.e., when the most effective strategy is expanded to other districts) and will identify organizational and clinician-level factors that affect SBIRT implementation and effectiveness. Design solutions that are responsive to key challenges and advantages of the study are detailed in
Box 1.
We will determine which study arm is superior according to implementation impact, effectiveness (clinical and cost), and implementation process. We chose the RE-AIM framework for aims 1 and 2 because it is widely studied and identifies five constructs that affect the quality, speed, and public health impact of efforts to translate research into practice: reach the intended target population; efficacy and effectiveness; adoption by target staff, settings, and institutions; implementation fidelity; and maintenance of intervention effects (
26,
27). For a broader understanding of the implementation process (aim 3), we chose the Consolidated Framework for Implementation Research (CFIR) (
28) to guide qualitative exploration of factors affecting implementation. CFIR synthesizes implementation science frameworks into five domains: intervention characteristics, outer setting (an organization’s economic, political, and social context), inner setting, characteristics of individuals involved in implementation, and implementation process.
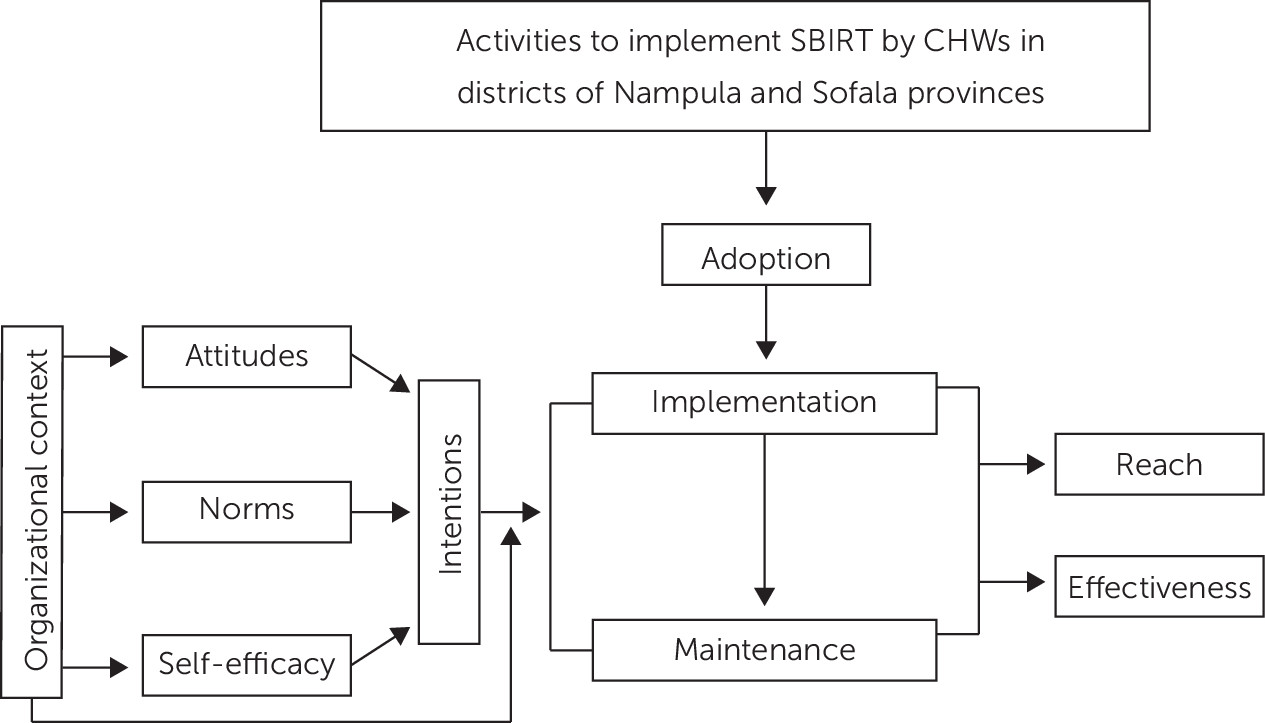
However, neither RE-AIM nor CFIR posit causal links among variables. Specifically, they do not describe how organization- and provider-level factors interact to affect implementation. To address this limitation, we borrow from and expand upon the work of Williams and Glisson (
Figure 1) (
29), who combined organizational factors with psychological variables from the theory of planned behavior (
25). We propose that the most proximal variable to CHWs’ and supervisors’ use of SBIRT is their intention to use it. Intentions are influenced by four determinants: attitudes (perceptions of SBIRT), descriptive norms (belief that people like me use SBIRT), injunctive norms (belief that important others expect me to use SBIRT), and self-efficacy (perception that providers can effectively perform SBIRT). We hypothesize that organizational factors influence the determinants of intentions and moderate the association between intentions and use of SBIRT. That is, even the strongest intentions may be thwarted if organizational resources do not support acting on those intentions. Our hypotheses are the following:
Hypothesis 1: RE-AIM Implementation Impact Evaluation
mSBIRT will yield significantly better RE-AIM implementation outcomes than will SBIRT-CTS. The primary outcome is reach (percentage of those in need who can access care). Secondary outcomes are adoption (percentage of clinic staff using SBIRT), implementation fidelity (adherence to model and strategy), and maintenance (use over 2 years) at months 12, 18, and 24.
Hypothesis 2: RE-AIM Clinical Effectiveness and Cost-Effectiveness Evaluation
First, clients exposed to mSBIRT will have greater reduction in hazardous drinking and improved health-related quality of life than those exposed to SBIRT-CTS at months 12, 18, and 24. Second, hazardous drinking reduction due to SBIRT will improve health-related outcomes, including HIV-related outcomes (e.g., reduced viral load) for people living with HIV at months 12, 18, and 24. Third, mSBIRT will be more cost-effective than SBIRT-CTS at months 12, 18, and 24.
Hypothesis 3: Implementation Process Evaluation
First, location of the organization (urban, suburban, or rural), culture, and climate will affect clinicians’ attitudes, perceived norms, and self-efficacy regarding SBIRT use, which will in turn affect intentions to use SBIRT. Second, clinicians’ demographic characteristics and intentions to use SBIRT will affect its adoption and use. Third, resources will moderate the association between clinicians’ intentions and implementation.
Methods
Overview
We obtained ethical approval from institutional review boards at New York State Psychiatric Institute and the Mozambican Institute for Health Education and Research. We will conduct a 2-year, cluster-randomized, hybrid implementation-effectiveness type-2 trial (
30) in 12 districts. CHWs will deliver the intervention in both intervention arms. A 2:1 randomization of districts will be used, with eight districts randomly assigned to mSBIRT and four to SBIRT-CTS. Randomization will be stratified on the basis of district size (i.e., number of CHWs) to ensure proper balance. The more effective strategy will be scaled up to the other arm in study year 3. Guided by the CFIR (
28), we will use mixed-methods assessments throughout the study to identify the factors affecting implementation. This study is embedded in a larger study of a scale-up of comprehensive mental health care (
31).
Study Sites
The study will be conducted in two Mozambican provinces—Nampula and Sofala. Nampula province is located in northern Mozambique, is its most populous province (5,758,920 inhabitants), and contains both vast rural areas and the country’s third-largest city. Sofala province (2,221,803 inhabitants) is in Mozambique’s central region. Research will be conducted in 12 districts (four assigned to SBIRT-CTS and eight to mSBIRT), which constitute approximately 60% of the provinces’ populations (i.e., 4,788,430 people). Every district comprises clinics with diverse degrees of urbanicity, including clinics that are rural, suburban, and urban. In both provinces, mental health services are presently provided only at district psychiatric clinics by one to two psychiatric technicians per district and one to five psychologists per province.
Each district has approximately seven primary care clinics staffed by two to five primary care providers. Approximately five CHWs per clinic attend to 250–400 families in their community annually, depending on each family’s health needs. The CHWs are the first level of contact in the community and are part of the National Mozambique Health System. They are involved in testing for and treatment of infectious disease (malaria, HIV, and TB) and in maternal-child health services.
Study Procedures
Overview.
Year 1 will be devoted to SBIRT implementation (
32), which will involve training and supervising staff, monitoring services and fidelity, providing implementation feedback to providers, and interviewing providers about implementation. In year 2, we will assess the sustainability of mSBIRT and SBIRT-CTS delivery (
32). These implementation and sustainability phases will occur within the context of the cluster-randomized trial. In year 3—the scale-up phase—the more cost-effective strategy will be implemented in districts that received the less effective strategy.
Regardless of the trial arm, Ministry of Health procedures require that individuals who are reached, in need, and provided with care be seen weekly and screened with SBIRT. Those who are reached and engaged in follow-up constitute a retained population. Each weekly contact with a provider is documented by the first two items from the AUDIT, the 12-Item Short Form Health Survey (SF-12) (
33), and the Mental Wellness Tool (mwTool, described below).
All patients and providers will provide written informed consent. Even though risks to provider participants are minimal, patient participants may experience increased distress and suicidal ideation or report physical or sexual abuse. In accordance with the Ministry of Health safety procedures, local coinvestigators (all clinicians) will be notified of an acute crisis within 24 hours. Additionally, we will convene a five-member data safety and monitoring board that will include an implementation scientist, statistician, mental health researcher with expertise in low-resource settings, Mozambique context expert, and ethicist. They will assist in monitoring adverse events and meet annually.
Implementation phase.
In the initial 6 months of the implementation phase, SBIRT and motivational interviewing experts will provide a 1-week training for Ministry Mental Health specialists. Trainees will present five cases in a group modality to become certified national trainers and supervisors. These newly minted trainers will then train and supervise the CHWs. We, the research team, will work with the trainers to adapt and pilot mSBIRT. We will also conduct a mixed-methods evaluation of the acceptability and feasibility of tablet use and capture CHWs’ intentions of using mSBIRT.
As part of the larger mental health study (
31), all CHWs will be trained to use a tablet-based, comprehensive mental disorders assessment tool—the mwTool—that identifies presence of substance use disorders, common mental disorders, severe mental disorders, and acute risk for suicide. We will measure trainees’ knowledge (information), attitudes toward people with hazardous drinking (motivation), and self-efficacy (skills) in providing SBIRT before and after training (
25,
29).
During the following 6 months of the implementation phase, the research team will provide active supervision through observation of role-play and case presentation sessions. CHWs will complete three cases and submit transcripts of the case sessions to their supervisor. Supervisors will evaluate the transcripts by using a treatment fidelity checklist comprising core intervention components and provide feedback to CHWs. If supervisors determine that a CHW conducts the SBIRT with insufficient fidelity or lacks competency, supervision and clinical support will continue until fidelity and competency are achieved.
The mSBIRT application software guides providers in following standardized treatment steps involving interactive methods, including knowledge tabs with tips and sample scripts, and automated processes to enable providers to recognize any clinical “red flags.” Metadata from the application will track individual provider implementation outcomes (adoption) and fidelity. Provider-level user data will indicate patients seen, key activity completion, and sessions per patient. If the user data of any provider appear to deviate from the standard sequence of activities, this will be flagged for supervisor intervention and corrective feedback to maximize fidelity. We will capture similar data in the SBIRT-CTS via provider and supervision checklists.
Sustainability phase.
The 12-month sustainability phase will begin once all CHWs and supervisors have achieved competency in either mSBIRT or SBIRT-CTS. We will monitor the CHWs, supervisors, and any Ministry of Health personnel involved in the study, but we will not provide supervision unless requested or deemed necessary on the basis of performance. We will sample six clinics in each arm from rural (N=2), suburban (N=2), and urban (N=2) areas. We will conduct 12 audio-recorded focus groups (1–1.5 hours each) with CHWs (one group per catchment area) and 24 semistructured household interviews with patients or their family members (two interviews per catchment area).
Scale-up phase.
In the scale-up phase, interviews will continue only in the six clinics of the superior arm: one focus group with CHWs at each clinic (N=6) and two household interviews per catchment area (N=12).
Screening and treatment.
The goal is for CHWs to use the mwTool to screen annually every household in their catchment area. For those who score positive for substance use disorders, CHWs will administer the AUDIT whose score will guide intervention. Patients with an AUDIT score of abstinence or low risk (<5) will receive feedback and follow-up in 4 weeks. Those with scores 5–15 will be considered to be at risk for or to have hazardous substance use and will receive a brief intervention weekly for 4 weeks. Patients who score in the harmful use range (AUDIT score ≥16) or in the dependent range (≥20) will also receive the four-session intervention plus medical monitoring by a primary care provider or psychiatric technician. Before the fourth session, CHWs will administer the AUDIT again. The fourth session reassessment will determine whether patients will be discharged (if they have an AUDIT score indicating abstinence or low risk), referred to an ongoing group intervention at the community level (AUDIT score is the same or improved, but not in low-risk range), or treated by a primary care provider or psychiatric technician (if they have worsening symptoms).
SBIRT sessions last 45–60 minutes and involve screening and a brief intervention consisting of providing feedback and health information, engaging patients in motivational conversations, and negotiating a plan for change (
34). SBIRT incorporates principles of motivational interviewing—using an empathic style, supporting motivation and self-efficacy for change (
35), building awareness about effects of alcohol use, and creating an action plan. We will establish a Mozambican SBIRT-mSBIRT workgroup with specialists who have expertise treating substance use disorders to adapt the treatment to the Mozambican context. Our adaptation workgroup will provide input into the mSBIRT application itself and suggestions for the general content and icon development.
Measures.
To determine the effectiveness and implementation outcomes, we will use the AUDIT and the mwTool. In both arms, the CHWs will screen household members yearly for mental health and substance use problems with the mwTool, which incorporates some AUDIT items as well as screeners for other mental health problems. Each CHW has a catchment area of 250–400 households (
36). To decrease CHW burden, the CHWs will administer the mwTool to only two household members (ages ≥16 years) per home but will ask about other household members. Local research has shown that surrogate respondents provide valid information on alcohol intake (
8). CHWs will individually administer the full AUDIT (
37) and SF-12 (
33,
38–
40) to anyone age ≥16 years screening positive for substance use disorders on the mwTool (
Table 2). On the basis of AUDIT scores, CHWs will deliver either mSBIRT or SBIRT-CTS, according to the trial arm. The full AUDIT will be administered at sessions 1 and 4 to measure effectiveness. The study measures are organized by RE-AIM outcomes (
Table 2) (
41–
55).
For aim 3, we will use a mixed-methods approach to identify organizational and clinician factors that affect SBIRT implementation. Interview guides and analyses will be guided by our conceptual model combining the theory of planned behavior with organizational constructs from the CFIR (
28). We will triangulate quantitative and qualitative data to identify the factors influencing adoption and sustainability and facilitate translation of research findings into effective policy. In the “crossover” phase, adoption and causal model measures will be administered to all participants. In each focus group, we will present our quantitative results in easy-to-interpret graphics and descriptions. We will ask participants to consider the results, adoption rate, and any associations between clinician (intentions) and organizational (culture, climate) factors and adoption.
Analysis Strategy
Hypothesis testing.
The cluster-randomized trial will address hypotheses 1 and 2 regarding the implementation and effectiveness of the interventions across the eight mSBIRT and four SBIRT-CTS districts. The hypotheses will be used to compare the interventions’ effect on outcomes measured for individuals and CHWs aggregated at the clinic level. We expect to include 62 clinics across the 12 districts, employing 417 CHWs who serve between 104,250 and 166,800 households. Clinic-level outcomes will be measured at baseline and then at 12, 18, and 24 months. Because clinics are nested within districts, we will use hierarchical linear models (HLMs), which will include random effects on each level and fixed effects for the intervention indicator and for the time variable.
Hypothesis 1 will test whether mSBIRT is superior to SBIRT-CTS on each of the RE-AIM implementation outcomes, by using HLM analysis, including an indicator for intervention, time (baseline, 12, 18, and 24 months), and intervention × time interaction. The magnitude and statistical significance of the interaction term will indicate whether a change in implementation outcomes over time significantly differs by intervention arm. If the arms differ on baseline measures, we will include those measures as control variables in the HLM.
Hypothesis 2 investigates the interventions’ effect on clinical outcomes (first and second subhypotheses) and on costs (third subhypothesis). For the first subhypothesis, we will use an HLM (controlling for intervention and time) to estimate changes in hazardous drinking and health-related quality of life at months 12, 18, and 24 after SBIRT exposure. Overall intervention effects will be tested over time, and interactions of the intervention by gender, age, and urbanicity of the patient will test for effect modification. For the second subhypothesis, we will again use an HLM (controlling for intervention and time) to predict changes in scores from baseline in health-related outcomes (viral load and HIV or TB care adherence) at months 12, 18, and 24 by using changes in hazardous drinking from baseline. We will assess effect modification by gender, age, and urbanicity by including them as interaction terms. Finally, the third subhypothesis will test whether mSBIRT is more cost-effective than SBIRT-CTS in improving both implementation (hypothesis 1) and clinical outcomes (first and second subhypotheses of hypothesis 2). Comparison of costs will be aggregated across the sustainability phase in year 2. We will assess health-related quality of life with the SF-12, which can be converted to construct quality-adjusted life years (QALYs) (
56). QALY is a measure of health-related quality of life that incorporates estimates of mortality and morbidity rates into a single index for ease of comparison across conditions and interventions. Incremental cost-effectiveness ratios will identify the superior arm for the scale-up phase (numerator=difference in mean costs between arms, denominator=difference in reach and reduction in hazardous drinking). Uncertainty in costs and outcomes will be assessed with cost-effectiveness acceptability curves (
57–
59).
Power analysis for hypotheses 1 and 2.
Power was calculated on the basis of participation of approximately 480 CHWs located across clinics in 12 districts and an anticipated dropout rate of about 20%. Each CHW visits between 250 and 400 families in the community at least once a year, resulting in a coverage of approximately 150,000 families (about 75,000 families within each randomized condition). For hypothesis 1, to test for differences in implementation outcomes between randomized study condition at the family level (e.g., percentage households screened), we will have 80% power to detect small to moderate effect sizes between the two conditions, with Cohen’s d=0.23–0.36, following standard power calculations for cluster-randomized trials (
60). This power calculation was derived by assuming an intraclass correlation (ICC) of families within the 12 randomized districts in the typical range for health care outcomes across randomized regions, that is, an ICC of 0.02–0.05 (
61,
62). For hypothesis 2, we conservatively assumed that 3%−5% of the population will be identified as having engaged in hazardous drinking and will be enrolled in either intervention. Thus, we expect that 4,500–7,500 clients will enroll across the two randomized study arms nested within 480 CHWs who will provide treatment. Given this sample of treated individuals, we will have 80% power to detect intervention differences of Cohen’s d ≥0.16. This power was calculated by assuming an ICC of 0.4 for client outcomes within CHWs, which is conservatively at the high end of ICCs found within health service providers in low-income settings (
63). All power calculations assume an alpha of 0.05 and that all tests are two-tailed.
For hypothesis 3, we will examine organizational and clinician-level factors affecting implementation of the intervention with data from the implementation, sustainability, and scale-up phases. The first subhypothesis of hypothesis 3 is that organizational factors such as urbanicity, culture, and climate will affect clinicians’ attitudes, perceived norms, and self-efficacy regarding SBIRT use, which will in turn affect their intentions to use SBIRT. The second hypothesis is that clinicians’ demographic characteristics and intentions to use SBIRT will affect SBIRT adoption and use. To test both subhypotheses, we will use structural equation modeling (
64,
65) to estimate the effect of organizational factors and clinicians’ demographic characteristics on determinants of intentions (attitudes, norms, and self-efficacy) and the effect on intentions (
Figure 1). Organizational factors will include urbanicity, number of staff, patients’ characteristics and the ready-set-change measure (
66). Consistent with best practice, we will aggregate these organizational measures at the clinic level. Intentions and determinants of intentions to adopt SBIRT will be measured with validated self-report measures (
Table 2). The use of structural equation modeling allows direct incorporation of clinic-level random effects and provides straightforward estimation of indirect effects. We will also use structural equation modeling to estimate the effect of intentions on adoption, while controlling for the clinic and clinician characteristics described above. We will use interaction terms to further test for effect modification of the organizational factors on the association between intentions and adoption.
For qualitative analysis, interviews and focus groups will be digitally recorded, transcribed, and loaded into Nvivo software for analysis. To avoid bias, analysts will be blinded to implementation outcomes. Initial coding will draw on the CFIR, and an inductive process of iterative coding will identify themes and categories. Analysts will then be unblinded to implementation outcomes to determine distinguishing constructs. Finally, for mixed-methods data analysis, quantitative data are gathered before qualitative data and weighted equally: QUAN→QUAL (
67,
68); the function is complementary, and the process is connecting (having the qualitative data set build on the quantitative data set) (
67).
Results
The more cost-effective strategy will be scaled up in the other districts by using procedures outlined in the implementation phase. However, during the scale-up phase, trained Ministry of Health personnel will be charged with delivery, training, and supervision of the superior strategy in all clinics without research team support. The scale-up phase is critical to identify facilitators and barriers through process evaluation and continued tracking of internal and external factors in clinics that started in the superior arm and those that switch strategies.
Next Steps
We will work with stakeholders from multiple sectors to use the lessons learned from our study to develop an implementation tool kit that can guide SBIRT for community-wide HDS scale-up and dissemination in LMIC countries, New York State, and city departments of health and mental health. We will review the data, create a draft tool kit, and present it to LMIC policy makers and WHO representatives. Policy maker feedback will help finalize the tool kit. This kit will be freely available to all research collaborators.