Research demonstrates that interventions targeting the early phase of psychotic illness reduce symptoms and hospitalizations and improve quality of life (
1). In the past 5 years, multicomponent coordinated specialty care (CSC) programs tailored to the needs of young people with recent-onset psychotic disorders have spawned across the United States. These programs provide psychopharmacological treatment, case management, family education and support, psychotherapy, primary care coordination, and supported education and employment services (
2). Despite improving symptoms and functioning compared with usual care (
1), these programs produce wide heterogeneity of response (
3).
A prominent aim of CSC is to alleviate symptom burden. High levels of positive and negative psychotic symptoms, and other related psychopathology, in early stages of psychosis may predict poor outcomes in remission, social and vocational functioning, and quality of life, even at 10-year follow-up (
4–
6). Identifying characteristics correlated with persistent psychopathology might help identify individuals who are less likely to respond to CSC earlier in treatment and could lead to developing and implementing approaches to enhance therapeutic response and person-centered care. This study aimed to determine the prevalence and predictors at baseline of severe persistent psychopathology among individuals with a recent-onset nonaffective psychotic disorder during the first year of enrollment in a large CSC program across New York State.
Methods
This study was conducted with data from OnTrackNY, a CSC program across 20 sites in community mental health programs and academic medical centers in New York State. The program enrolled patients ages 16 to 30 who had experienced a nonaffective, psychotic disorder for less than 2 years. Exclusion criteria included autism spectrum disorder, serious or chronic medical illness, substance-induced psychosis, affective psychosis, psychosis due to a general medical condition, and intellectual disability (IQ <70) (
7).
Primary clinicians, master’s- or doctoral-level licensed practitioners, assess participants on the basis of all available information from the individual, their family, and other team members at admission and at quarterly follow-ups. Our sample included all 1-year eligible participants with at least one follow-up who entered the program between 2013 and 2018. To provide a sufficient period for individuals to respond to treatment, we selected a 1-year time frame. The use of deidentified data for research purposes was approved by the New York State Psychiatric Institute Institutional Review Board.
Our main outcome measure was level of symptoms, measured by the Mental Illness Research, Education, and Clinical Centers Global Assessment of Functioning scale (MIRECC GAF) (
8). The MIRECC GAF was delivered at baseline and at each quarterly follow-up assessment. The scale is scored from 0 to 100, with lower scores indicating greater symptom severity. Symptom types include suicidality, mood, anxiety, and psychosis. Accuracy based on expert-rated vignettes was high for MIRECC GAF symptom scoring, with 89% of 63 OnTrackNY clinicians scoring within 10 points of the gold standard, determined by expert raters who mapped vignette details to specific criteria within the chosen decile (
3).
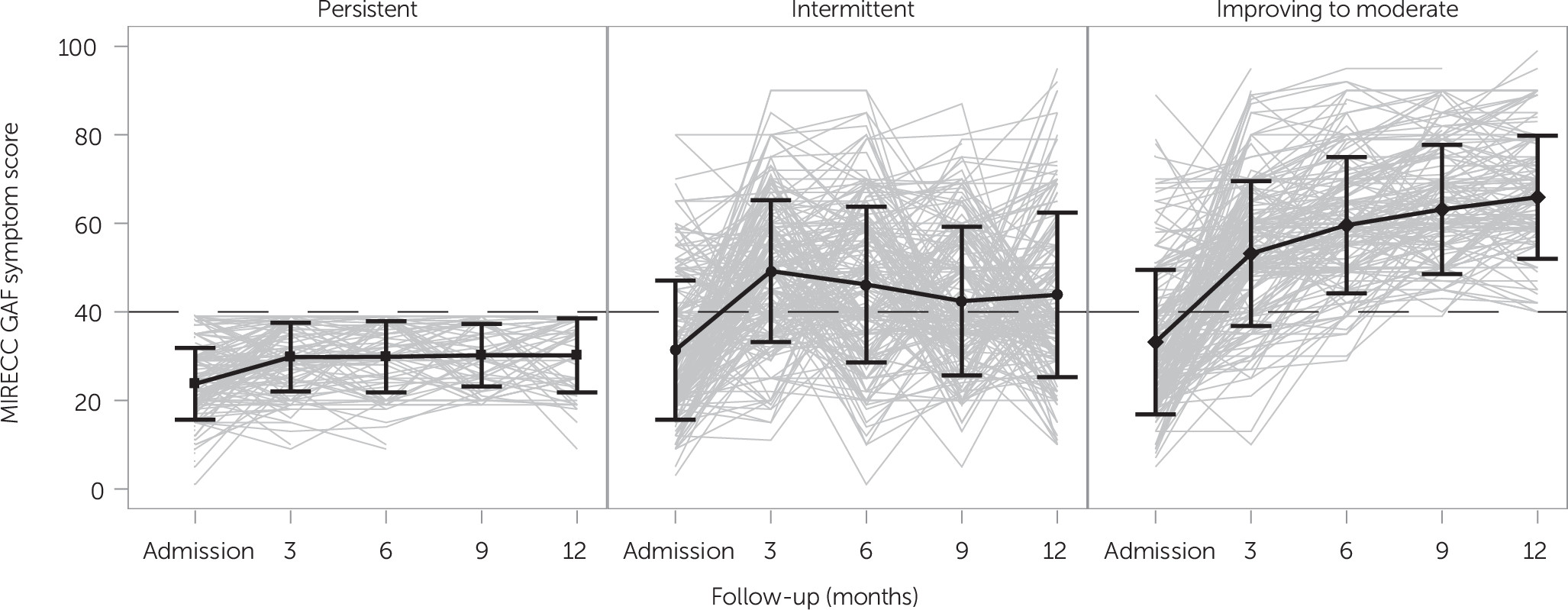
Three symptom groups were constructed on the basis of a MIRECC GAF score threshold of 40. A score of less than 40 reflects impairments in reality testing or communication (delusions; intrusive hallucinations; illogical, irrelevant, or obscure speech), suicidal preoccupation, or dangerousness to self or others. Scores above 40 may reflect serious symptoms, such as suicidal thoughts, severe obsessions, or anxiety, but no impairments in reality testing. The symptom groups included “persistent,” comprising individuals who never scored above the threshold; “intermittent,” comprising individuals who scored above 40 at one point (baseline or follow-up) but then fell below the threshold at least once during follow-up; and “improving to moderate,” comprising individuals who always scored above the threshold or improved to score above the threshold during follow-up.
Baseline predictors were categorized into demographic characteristics, family and living situation, clinical measures, and pathways to care. The baseline assessment covered the period between program enrollment and the 90 days prior. Demographic characteristics included age, gender, race-ethnicity, highest level of education, and insurance status. Family and living situation included homelessness, current living situation, preference for family involvement, current family contact, and fiscal support from family.
Clinical variables included primary diagnosis (schizophrenia, schizoaffective disorder, schizophreniform disorder, other); current antipsychotic prescription and, in case of current prescription, whether the participant had adherence to medication of at least 80% (not medication adherent, adherent, not prescribed, unknown) at baseline; and cannabis use (yes, no). Level of functioning was measured with the MIRECC GAF occupational and social functioning subscales (
8). Days from onset of psychotic symptoms to first mental health contact (help-seeking duration of untreated psychosis [DUP]), days from onset to program enrollment (CSC DUP), days from first mental health contact to program enrollment (referral DUP), and treatment history (type of first mental health contact, referral source, previous treatment, and service use) were also included.
Differences in baseline characteristics by symptom group were described by using means and standard deviations for normally distributed continuous measures, proportions for categorical measures, and medians and interquartile ranges (IQRs) for skewed continuous measures. Differences were assessed by using one-way ANOVAs, chi-square tests, or a nonparametric Kruskal-Wallis test to match the baseline characteristics’ distribution. Post hoc pairwise differences in comparison to the persistent group were computed if overall differences were significant at the 5% level. Given the exploratory nature of these analyses, tests were not corrected for multiple comparisons.
All hypothesis tests were two-sided, with a significance level of 5%, and all analyses were done with SAS, version 9.4. Few MIRECC GAF symptom scores were missing: 1% of values were missing at admission, and 4%, 5%, 5%, and 6% were missing at 3-, 6-, 9-, and 12-month follow-up, respectively. Additionally, of the participants with at least one follow-up, 23% (N=260) were discharged prior to 1 year. (Results of a sensitivity analysis using the subset of clients who were enrolled for at least 1 year [N=869] are provided in an
online supplement to this report.)
Results
Of eligible participants in their first year of enrollment with at least one follow-up (N=1,129), 12% (N=141) were persistently symptomatic, 21% (N=235) were in the intermittent group, and 67% (N=753) were in the improving-to-moderate group. Individual-level MIRECC GAF symptom score trends within each group are depicted in
Figure 1. (Baseline measures by symptom group are presented the
online supplement.)
At baseline, participants in the persistent-symptom group were more likely to be uninsured compared with those in the intermittent and the improving-to-moderate group (10% [N=14] versus 3% [N=8] and 4% [N=30], respectively), a difference that was no longer significant in sensitivity analyses. Participants with persistent symptoms were also more likely to be homeless compared with those in the improving-to-moderate group (11% [N=16] versus 4% [N=28]). There were no significant group differences in gender, race, age, highest level of education, or family contact or living situation.
The persistently symptomatic group had a greater percentage of participants who were not adherent to prescribed antipsychotic medication at baseline (N=31, 22%) compared with the intermittent (N=29, 12%) and improving-to-moderate (N=86, 11%) groups. Persistently symptomatic participants were more likely to have a baseline diagnosis of schizophrenia than those in the improving-to-moderate group (39% [N=55] versus 27% [N=201]). The persistently symptomatic group also reported more baseline cannabis use than the improving-to-moderate group (45% [N=64] versus 37% [N=275]), but this difference no longer appeared in sensitivity analyses. Although they were statistically significant, differences in mean baseline MIRECC GAF occupational or social functioning scores were not clinically meaningful (<10-point difference).
A longer time from onset of symptoms to program enrollment (CSC DUP) was found among the persistently symptomatic group (median=223 days, IQR=101–382 days) compared with the improving-to-moderate group (median=147 days, IQR=76–310 days). This finding was in part driven by a significantly longer duration between first mental health contact and program admission (referral DUP) among the persistently symptomatic group (median=83 days, IQR=36–262 days) compared with the improving-to-moderate group (median=70 days, IQR=32–171 days). The percentage of people with a previous psychiatric hospital admission was similar across symptom groups.
Discussion
This study aimed to estimate the prevalence and identify the predictors of persistent symptoms of young people enrolled at 20 CSC sites across New York State. We found that 12% of participants remained severely symptomatic over a 1-year follow-up. Approximately 21% had intermittent symptoms, while 67% had a relatively improving-to-moderate course. Being nonadherent to medication, being homeless, receiving a diagnosis of schizophrenia at baseline, and a longer time from symptom onset and program enrollment were associated with persistent symptoms.
We found a prevalence of symptom persistence at the lower range of previous studies, which identified persistent symptoms in 16%−27% of young people enrolled in intensive treatment programs for early psychosis during follow-up periods of similar length, even though these studies focused specifically on persistence of psychotic symptoms (
4,
9–
12). In line with previous research, we found that medication nonadherence (
13,
14), having a diagnosis of schizophrenia, and longer delays between onset of psychotic symptoms and treatment initiation (
9,
14,
15) were associated with poor symptomatic outcome.
Young persons who were homeless were more likely to have persistent psychopathology. Together with the association between persistent symptoms, longer time to program enrollment, and medication nonadherence, this finding suggests a pattern of barriers in access to care, lack of stability, and basic resources during the early phase of treatment enrollment among a subgroup of young people. These findings held in sensitivity analyses. These identified baseline predictors of persistent symptoms may prevent participants from optimally benefiting from intensive treatment like CSC.
It is possible that certain individuals may need additional support to meet basic needs beyond mental health treatment that CSC is unable to provide (e.g., housing opportunities, family structure, higher-paying jobs to address societal barriers). Longer DUP was driven by longer duration between first mental health contact and program admission, which could mean that a greater effort for earlier referral and admission to CSC could be beneficial. Regarding pharmacological treatment, a post hoc analysis showed that 10% (N=14) of the participants in the persistent group were prescribed clozapine at least once, with a similar number in the intermittent group, and 5% (N=38) of the improving-to-moderate group. These findings indicate potential room for improvement regarding a faster introduction of strategies and pharmacological therapies shown to improve symptomatic burden.
Although this study does not allow any claims about causality, the pattern of findings suggests that persistence of symptoms may be attributable to medication nonadherence, treatment resistance, and various other interacting factors that may impede clinical improvement, including circumstances related to access to care and basic needs. Future work should investigate the interplay and time-varying nature of these factors to better understand the mechanisms driving symptom persistence.
This study had several limitations. First, 23% of our sample with at least one follow-up disengaged prior to 1 year. Although a disproportionate number of these participants were in the group that initially was persistently symptomatic, we have no data on participants’ symptoms postdischarge and thus cannot predict whether early discharge is associated with symptoms and could thus represent missing data in a population with nonrandom characteristics. However, sensitivity analyses showed mostly similar results. Second, although some studies show that most changes occur in early stages of treatment, 1 year might not be sufficient because the CSC model is designed to provide treatment for an average of 2 years. Third, data were collected by clinicians, which can introduce clinician bias. Fourth, the symptom subscale does not allow for exploration of specific symptoms, and we could not disentangle psychotic symptoms from suicidality and other forms of psychopathology. Finally, OnTrackNY does not include all people with recent-onset psychosis in New York State. The generalizability of the study findings to other health care systems in the United States may also be limited.
Conclusions
Twelve percent of participants remained highly symptomatic during 1-year follow-up after enrollment in a CSC for early psychosis in New York State. These participants were more likely to be medication nonadherent, be homeless, receive a schizophrenia diagnosis, and have a longer time to program enrollment. The pattern of findings may imply that certain individuals need additional resources to fully benefit from intensive treatment programs offering CSC. In addition, outreach efforts should target health settings to reduce referral DUP to CSC programs. In turn, these programs should be vigilant in identifying this subpopulation as early as possible and intensifying treatment to encourage medication adherence and promote interventions that enable participants to profit from specialized services.