Polypharmacy, defined as the concurrent use of two or more psychotropic drugs, is common among patients with schizophrenia (
1). Treatment with a single neuroleptic is commonly associated with persistent psychotic symptoms, but other clinical features such as depression and anxiety are also prevalent and may be treated with other psychotropic medications (
2). Although polypharmacy is not contraindicated, the practice increases both the costs of care and the possibility of untoward side effects through pharmacokinetic or pharmacodynamic interactions or both, and it often leads to individual doses well in excess of therapeutic guidelines (
3).
Clozapine, with its superior efficacy in treatment of refractory schizophrenia, its broader spectrum of symptom control, and its lack of extrapyramidal side effects (
4), might be expected to decrease the number and type of concomitant medications. In one of the few studies assessing the impact of clozapine on clinicians' prescribing patterns, Drew and associates (
5) reported a reduction in the concurrent use of two neuroleptics following the initiation of clozapine among 11 patients with schizophrenia.
We surveyed prescribing patterns for a group of outpatients with schizophrenia before and after the initiation of clozapine. We hypothesized that clozapine initiation would be followed by a decrease in use of concomitant medication.
Methods
Subjects were outpatients with a DSM-IV diagnosis of schizophrenia who received a prescription for clozapine between November 1997 and January 1998 at the Clarke Institute of Psychiatry, an academic psychiatric center in metropolitan Toronto. Computerized pharmacy records generated data on prescribing patterns, and further information was obtained from case records of the identified patients and from the attending psychiatrists.
For each patient the following data were collected: age, gender, duration of illness, and psychotropic medications prescribed one month before the initiation of clozapine, as well as the duration of use and daily dose of clozapine and all medications used concomitantly with clozapine. Antipsychotic drug doses were converted to chlorpromazine equivalents, using established guidelines (
6). For patients who were receiving depot medications, we used Foster's method (
7) for converting doses.
Results
The 42 patients identified by the computerized search included ten female patients (24 percent) and 32 male patients (76 percent). Their mean age was 34.2±8.3 years, with a range from 21 to 55 years. The mean duration of illness was 13.1±7.7 years, with a range from three to 36 years.
As
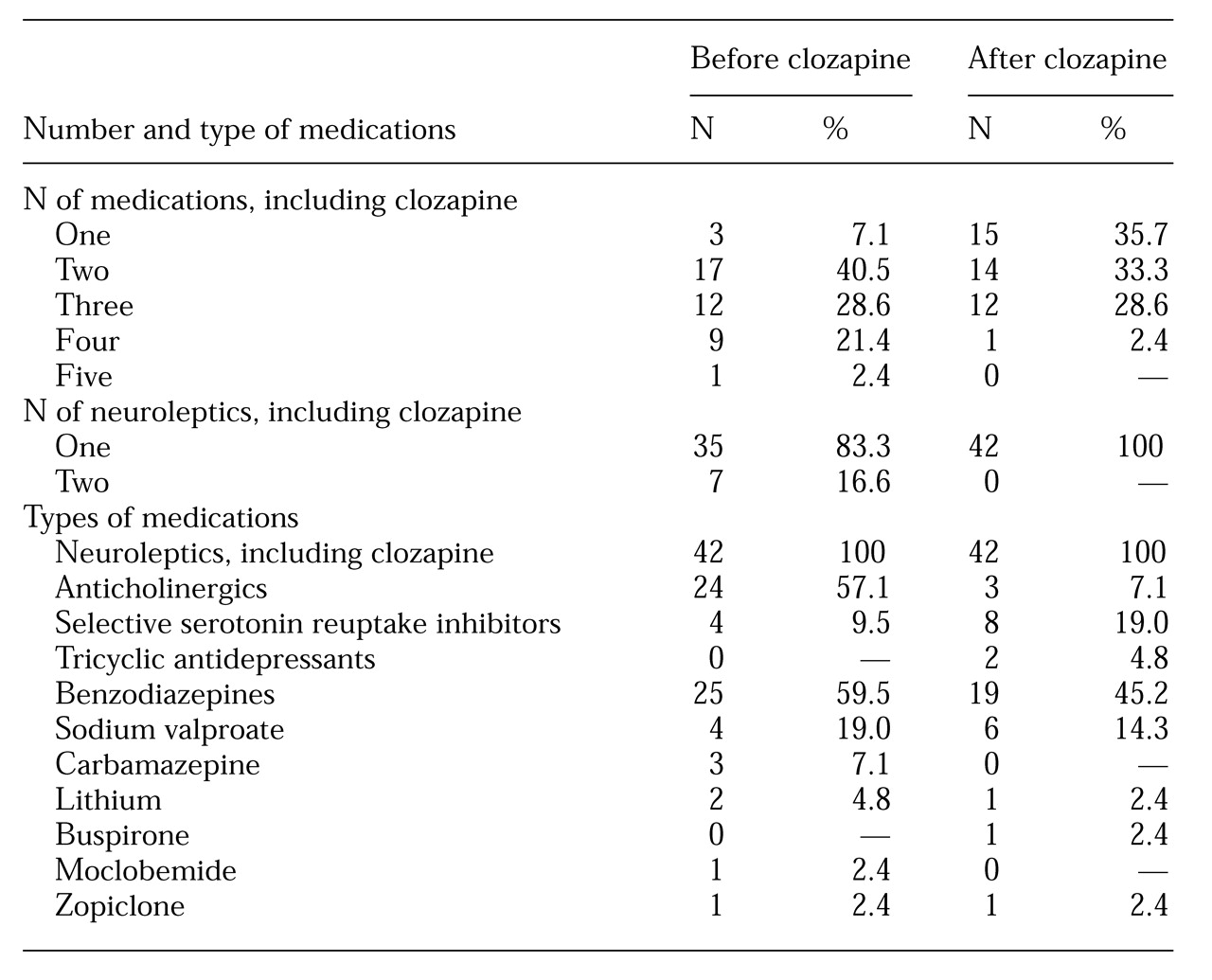
Table 1 shows, after the initiation of clozapine, the number of patients taking two or more drugs decreased by 31 percent, from 39 to 27, and the number of patients taking a single psychotropic agent increased five times, from three to 15. The number and types of concomitant medications used before and after initiation of clozapine are also outlined in
Table 1.
The mean number of medications as well as the mean number of neuroleptics was significantly different before and after clozapine. The mean number of medications decreased from 2.8±.97 before clozapine to 2±.87 after clozapine (t=4.22, df=41, p<.005). The mean number of neuroleptics decreased from 1.2±.4 before clozapine to 1±.4 after clozapine (t=5.9=87, df=41, p<.005). The patients received clozapine for a mean of 2.4±1.4 years, with a range from .3 to 5.7 years.
Patients' mean current daily dose of clozapine was 410.7±157.1 mg, with a range from 75 to 900 mg. This amount was equivalent to 809.5± 316.8 mg per day of chlorpromazine, with a range from 150 to 1,800 mg. The average chlorpromazine-equivalent dose of neuroleptic drugs before clozapine was 539.3±443.7 mg per day, with a range from 50 to 1,567 mg. The reduction in the mean chlorpromazine-equivalent dose of neuroleptic before and after clozapine was significant (t=3.8, df=41, p<.05).
No significant differences were found between the male and female patients in either the mean daily dose of clozapine (397±145.1 mg for male patients and 425.5±193.5 mg for female patients) or in the mean daily dose of chlorpromazine-equivalent neuroleptic before initiation of clozapine (527.1±457.9 mg for male patients and 578.4±415.1 mg for female patients). Six of eight patients receiving a selective serotonin reuptake inhibitor (SSRI) had concurrent depressive symptoms, one had obsessive-compulsive symptoms, and the eighth received fluvoxamine to elevate the plasma clozapine level.
Discussion and conclusions
After initiation of clozapine, none of the patients in the sample were receiving a concomitant neuroleptic. This finding differs from results of studies in Europe, where the combined use of clozapine and typical neuroleptics has been reported for 23 percent to 35 percent of patients (
8,
9). This difference in prescribing pattern may reflect the differing beliefs of the physicians. Physicians who combine clozapine with typical neuroleptics may believe that this practice results in additional antipsychotic efficacy in treating patients with less than optimal response to clozapine alone.
As expected, the use of anticholinergic agents dropped after the introduction of clozapine. A smaller reduction in benzodiazepines, the most common group of drugs prescribed concomitantly with clozapine, was also noted, possibly because of the sedative effect of clozapine. Carbamazepine, used by three patients before initiation of clozapine, was discontinued in all three cases after clozapine was started, most likely because of the increased risk of agranulocytosis when the two medications are used together (
6). Carbamazepine was replaced with sodium valproate, which accounted for its increased prescribing prevalence.
A twofold increase in the concomitant use of SSRIs occurred; 75 percent of the subjects who took SSRIs received them for concurrent depressive disorder. SSRIs are considered to be a better choice than tricyclic antidepressants because of the additive anticholinergic and epileptogenic effect with combinations of clozapine and tricyclics (
10).
Although our findings indicated an increase in the number of patients receiving monotherapy, the use of concomitant medications was still common, with 64 percent of the patients receiving two or more drugs. Other studies have also reported this trend. Among 656 patients in Denmark who were receiving clozapine, 35 percent were receiving concomitant neuroleptics, 28 percent benzodiazepines, 19 percent anticholinergics, 11 percent antidepressants, 8 percent antiepileptics, and 2 percent lithium (
9). Joffe and associates (
8) reported that 41 percent of the 39 clozapine-treated outpatients in their study were also taking other drugs; 23 percent received additional neuroleptics, and 17 percent were taking other medications, usually benzodiazepines.
Our findings, which are based on 42 patients attending an academic psychiatric center, may not be representative of all patients receiving clozapine. Nonetheless, the data show a trend toward reducing the multiplicity of drugs after initiation of clozapine. At the same time, however, polypharmacy persisted in a number of cases. The efficacy and safety of using other psychotropic drugs need to be further investigated systematically, and clinicians must bear in mind the possible drug-drug interactions that can occur between clozapine and other medications. Although monotherapy in the pharmacological management of schizophrenia is frequently not achievable, the rationale for adding or continuing concomitant psychotropic medications must be continuously reviewed.
Acknowledgment
The authors thank Gordon Parker, M.D., Ph.D., for his comments.