Understanding of the diagnosis and treatment of posttraumatic stress disorder (PTSD) has recently matured to the point that it has been possible to derive treatment guidelines from research evidence. Guidelines developed by the International Society for Traumatic Stress Studies (ISTSS) (
1) and derived from surveys of expert clinicians and researchers (
2) recommend medication and specific cognitive-behavioral therapies as first-line treatments. The recommendation that selective serotonin reuptake inhibitors (SSRIs) be used as first-line medication treatment is supported by large multicenter randomized controlled trials (
3,
4,
5,
6) that led to the approval of sertraline and paroxetine for PTSD by the U.S. Food and Drug Administration (FDA).
Smaller randomized controlled trials had previously supported the efficacy of the traditional categories of antidepressant medications—tricyclics and monoamine oxidase inhibitors (MAOIs)—for the treatment of PTSD (
7,
8). These agents are not recommended as first-line options in PTSD guidelines (
1,
2), principally because of their side effect profiles and because of safety concerns. Rather, these and other medications are recommended as second-line strategies—treatments that can be substituted for or added to first-line interventions when the initial clinical response is inadequate or when tolerability is an issue. Second-line medication interventions for PTSD, apart from traditional antidepressants, include novel antidepressants other than the SSRIs—for example, nefazodone, bupropion, and mirtazepine—and mood stabilizers.
Support for the use of these agents is not considered as compelling as that for the first-line agents, because evidence of efficacy typically falls short of the standard of randomized controlled trials (
1). The one published randomized controlled trial of benzodiazepines for the treatment of PTSD did not support efficacy (
9). Adverse consequences of benzodiazepine treatment among patients with PTSD have been reported (
10), and their use for PTSD is not recommended by the ISTSS guidelines (
1). The newer antipsychotic medications have only recently been evaluated in PTSD trials. The guidelines recommend that their use be reserved for second-line or adjunctive interventions when patients do not respond to other treatments (
1) and for explosive, irritable, aggressive symptoms and violent behavior (
2).
Recent information about prescribing patterns for patients with PTSD is limited. Reports from multisite samples from Department of Veterans Affairs (VA) specialized PTSD programs document prescribing rates for benzodiazepines of 36 percent (
11) and rates for antipsychotic medications of 9 to 10 percent (
12). These data were collected in the early 1990s, before the general availability of newer antipsychotic agents associated with a lower risk of tardive dyskinesia, and the authors note a rate of antipsychotic prescribing of 26 percent for a later period at their treatment site (
13). Thus the use of benzodiazepines and possibly of antipsychotics appears to be at variance with treatment recommendations in VA populations.
These two reports focus only on specific medication categories and refer to the predominantly male subpopulation of veterans with PTSD receiving treatment through VA programs. We are not aware of any reports of prescribing trends in nonveteran populations of persons with PTSD. A broader view is of interest given that nonconformance with guidelines may reflect inadequate dissemination of knowledge gained from research or limitations of research evidence and first-line interventions.
The aim of this study was to comprehensively evaluate medication treatment in a community-based population of persons with PTSD—with women well represented—by analyzing state Medicaid prescription data. A specific aim was to determine whether the trends noted in VA populations—frequent use of antipsychotic and anxiolytic or hypnotic medications—would be apparent in the New Hampshire Medicaid population of persons with PTSD. PTSD often co-occurs with other disorders, particularly major depression, and the two are treated with overlapping medications. Therefore, in addition to reporting prescribing frequencies for medications from different classes, we compared prescriptions for patients with PTSD and those for patients with major depression alone or in combination with PTSD to determine relationships between prescribing and a diagnosis of PTSD.
Methods
This analysis was reviewed and approved by human subjects protection committees for the New Hampshire Department of Health and Human Services and for Dartmouth Medical School as part of a larger study of behavioral health treatment for Medicaid beneficiaries. Because data were retrospectively analyzed from a deidentified administrative database, informed consent was not obtained. We used all paid Medicaid claims from December 1999 for New Hampshire beneficiaries between the ages of 18 and 65 years. Medicare claims diagnoses were used to supplement Medicaid diagnoses when beneficiaries had dual eligibility. Diagnoses for the group were surveyed between January and December of 1999 to identify categories that may have been coded in one month and not another.
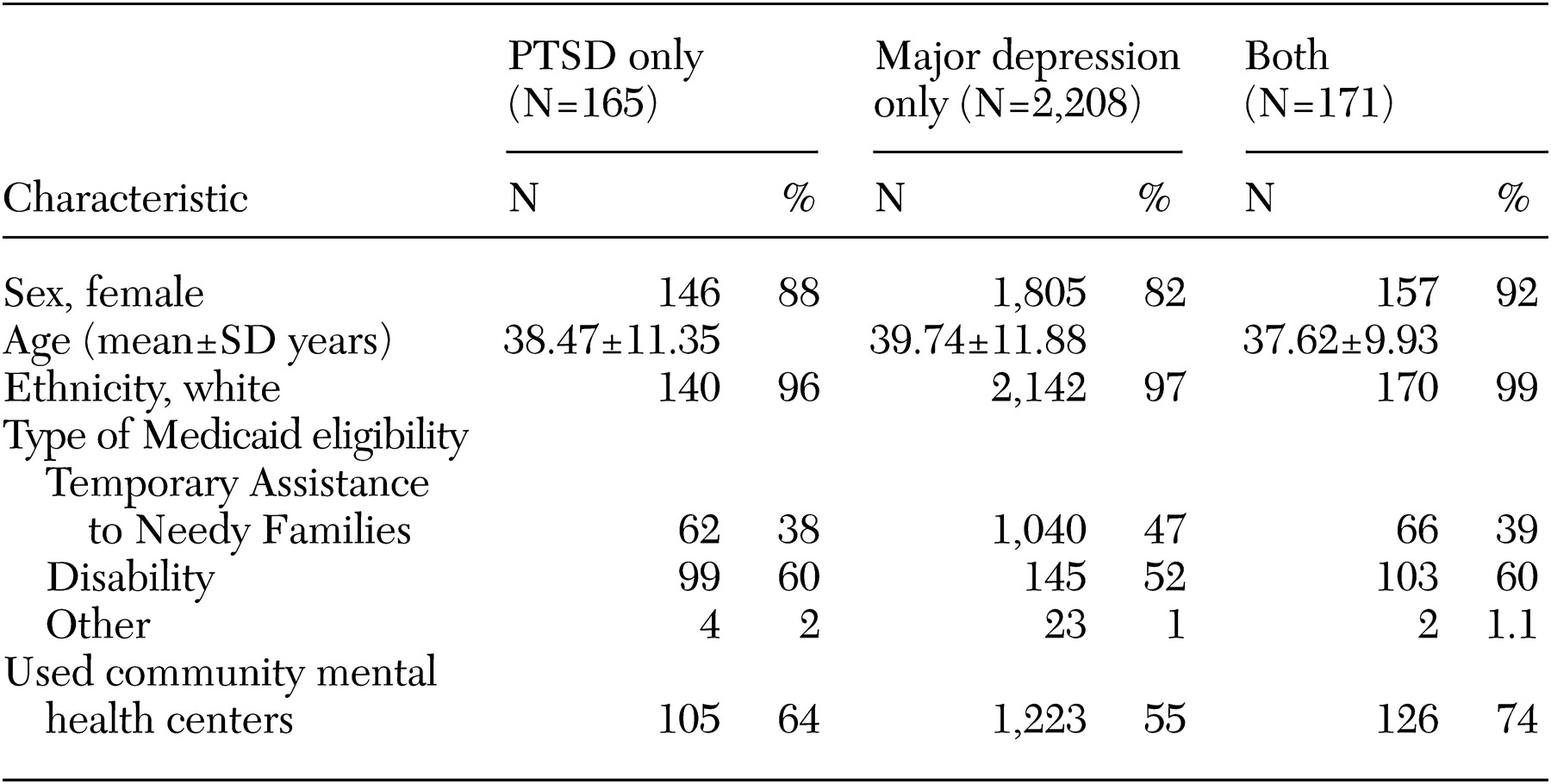
A total of 443 patients with ICD-9 diagnostic coding for PTSD were identified. For 165 of these patients, PTSD was the only psychiatric diagnosis coded. The most common co-occurring diagnosis among persons for whom PTSD was also diagnosed during the year was major depression (171 patients), followed by bipolar disorder (34 patients) and other anxiety disorders (neurotic disorders per ICD-9) (25 patients). Because of the relatively small number of cases in the other categories, and for the reasons outlined above, we focused on PTSD, major depression, and major depression co-occurring with PTSD. Demographic information for each group was obtained, including gender, age, race, type of Medicaid eligibility (most often Temporary Assistance for Needy Families or disability), and whether treatment was received in a community mental health center.
The tabulation of medication was cross-sectional. Nonduplicate paid prescription claims for psychotropic medications during December 1999 were identified. The mean number of psychotropic medications and the frequencies of prescription for specific medication categories were tabulated for each diagnostic group. The medication categories were SSRIs, other novel antidepressants (mirtazepine, nefazodone, and bupropion), traditional antidepressants (tricyclics and MAOIs), trazodone (given that it is often prescribed as a hypnotic), atypical and conventional antipsychotic medications, mood stabilizers (lithium and anticonvulsants), and benzodiazepines and related hypnotics, a category that included the newer benzodiazepine receptor agonist hypnotics. We compared means and frequencies of prescriptions between the 165 persons with PTSD only and 2,208 patients with major depression alone as well as the 171 patients with both PTSD and major depression by using t tests and chi square statistics.
Results
Demographic characteristics
The patients' demographic characteristics are summarized in
Table 1. A majority of the study patients were young white women. Most of those with a diagnosis of PTSD were designated as disabled and were being treated in community mental health centers. About half of those with a diagnosis of major depression received care in community mental health centers. Because of the possibility that prescribing trends were influenced by setting, we conducted secondary analyses involving only the patients who received treatment at community mental health centers.
Medications
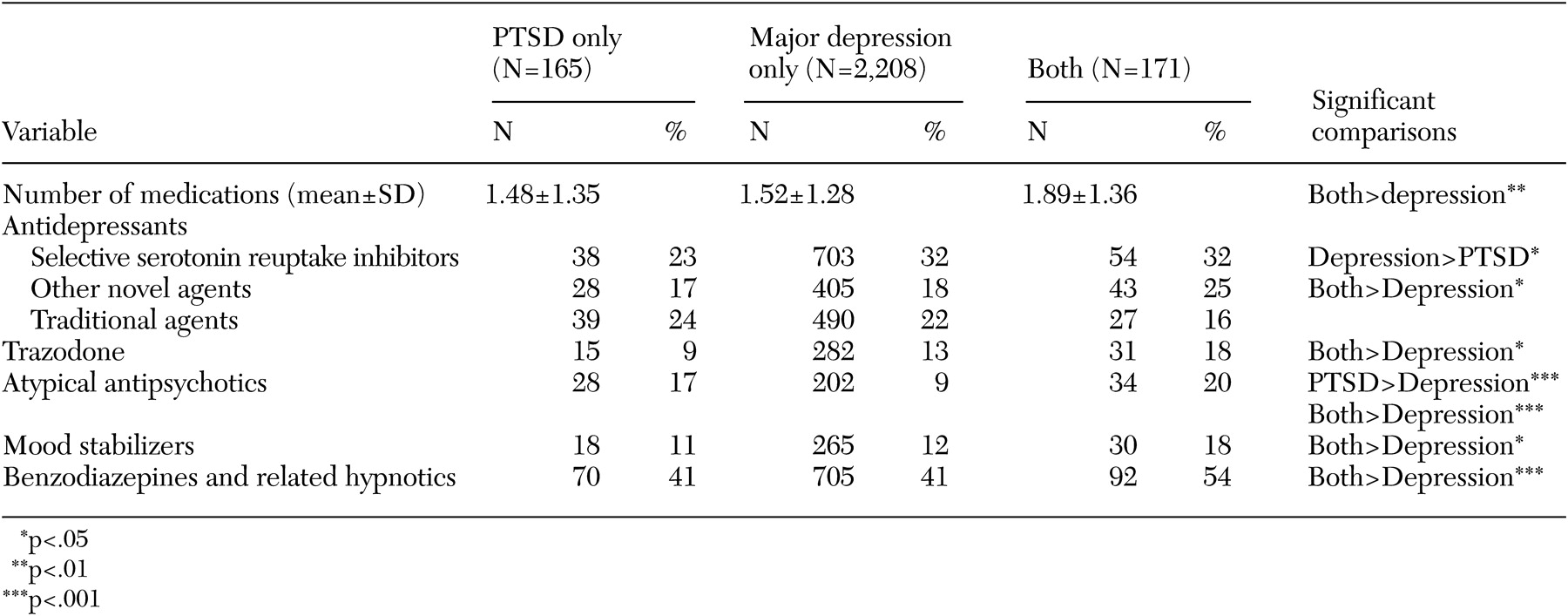
Prescription data for psychotropic medications and significant contrasts between patients with PTSD and patients with major depression with or without PTSD are presented in
Table 2. Most of the patients in each group received prescriptions for psychotropic medication during the month (127 patients, or 77 percent, in the PTSD group; 1,811 patients, or 82 percent, in the major depression group; and 152 patients, or 89 percent, in the group with major depression and co-occurring PTSD).
Patients with both PTSD and major depression received prescriptions for a greater number of psychotropic medications than those with major depression alone (t=3.45, df=2,378, p<.01). Patients with PTSD alone received prescriptions for SSRIs less frequently than patients with major depression alone (χ2=5.55, df=1, p<.02). Patients with major depression co-occurring with PTSD received prescriptions for non-SSRI novel antidepressants, atypical antipsychotics, trazodone, benzodiazepines and related hypnotics, and mood stabilizers more frequently than patients with major depression alone (χ2=4.81, df=1, p<.03 for novel antidepressants; χ2=3.99, df=1, p<.05 for trazodone; χ2=20.47, df=1, p<.001 for atypical antipsychotics; χ2=4.5, df=1, p<.03 for mood stabilizers; and χ2=10.7, df=1, p<.001 for benzodiazepines). Prescriptions for atypical antipsychotics were more common among patients with PTSD alone than among those with major depression alone (χ2=10.73, df=1, p<.001).
Treatment at community mental health centers
Almost all the categories of medication were prescribed more frequently in community mental health centers than in other settings. Some of the above-mentioned relationships were also observed in comparisons of only the recipients who received treatment at community mental health centers. Patients treated in community mental health centers who had PTSD alone were more likely to receive prescriptions for atypical antipsychotics than their counterparts who had major depression alone (26 of 105, or 25 percent, compared with 180 of 1,223, or 15 percent; χ2=7.44, df=1, p<.01). Community mental health center patients who had both major depression and PTSD were more likely to receive prescriptions for atypical antipsychotics (31 of 126, or 25 percent) than those with major depression alone (183 of 1,223, or 15 percent (χ2=8.60, df=1, p<.001) and were less likely to receive traditional antidepressants (17 of 126, or 13 percent, compared with 279 of 1,223, or 23 percent; χ2=5.79, df=1, p<.02).
Discussion and conclusions
As with studies of male veteran populations (
11,
12), our study documented frequent use of benzodiazepines and antipsychotics, which does not accord with strict interpretation of evidence-derived and expert consensus guidelines (
1,
2). Some of the differences between prescription rates for PTSD versus depression in our sample appear to have been influenced by the fact that a greater proportion of the patients with PTSD received care in community mental health centers.
Interpretation of our findings needs to account for the significant limitations that are inherent in the type of data used. Because trauma histories may not be routinely elicited, PTSD may not have been coded in some cases in which it was present. We also could not verify that coded diagnoses conformed to diagnostic criteria or that all relevant psychiatric diagnoses were represented. PTSD and major depression may be coded when a complex diagnostic spectrum is present that could include criteria for psychosis, dissociation, and personality disorder. The medication data were cross-sectional, so we could not determine what sequences of treatment preceded these medication regimens, and we do not have information about the clinical outcomes of the treatments.
In addition, these data reflect practices in December 1999. Patterns may have evolved since then. Although our data may be unique in the sense that they represent women in a nonveteran population, this demographic aspect of the sample also serves to limit generalizability. New Hampshire has ten community mental health center organizations, most of which are in rural settings. Although nurse practitioners are utilized, a majority of prescribers are psychiatrists. The vast majority of recipients are white. The preponderance of women in our sample appears to have been influenced by the greater prevalence of PTSD and major depression among women and women's greater likelihood of receiving Medicaid benefits through temporary assistance or disability.
Limitations notwithstanding, we believe that our findings provide an informative snapshot and raise issues that warrant scrutiny. First, the use of benzodiazepines and related hypnotics was prevalent and more frequent among patients with PTSD than among those with major depression without PTSD. It is likely that anxiolytic or hypnotic agents are prescribed in response to anxiety symptoms and sleep disturbances that frequently accompany PTSD. Although some possible benefit for associated symptoms cannot be excluded, available evidence does not support the efficacy of these agents for PTSD symptoms (
9), and greater risk of withdrawal and disinhibition with benzodiazepines among persons with PTSD has been suggested (
10). Greater use of trazodone for patients with both PTSD and major depression also probably reflects the indication of sleep disturbance, whereas the greater use of mood stabilizers may reflect a number of other target symptoms, including impulsivity and mood lability.
Second, although SSRIs are the only category of medications with FDA approval for the treatment of PTSD and the most substantially supported by randomized controlled trials, their use was less common among patients in our study with PTSD than among those with major depression. Non-SSRI novel antidepressants were more frequently prescribed for PTSD co-occurring with major depression. These two observations suggest that SSRIs were not being prescribed preferentially for PTSD, which appears discrepant with recommendations for SSRIs as first-line interventions and may reflect lack of knowledge about the strength of efficacy data.
Alternatively, residual symptoms may be driving practitioners to switch to or add second-line agents. Bupropion was the most common (39 percent) of the non-SSRI novel antidepressants prescribed for PTSD alone, and venlafaxine was the most often prescribed for major depression co-occurring with PTSD (37 percent). Rates for nefazodone and mirtazepine ranged between 14 and 25 percent of the non-SSRI novel antidepressants prescribed for PTSD and major depression with PTSD. This pattern does not suggest a uniform indication.
Finally, atypical antipsychotic medications were more frequently prescribed for patients with PTSD alone or in combination with major depression. Published randomized controlled trials addressing the use of atypical antipsychotics for PTSD appear to be limited to two relatively small recent studies of olanzapine, with mixed results regarding efficacy (
13,
14). The not uncommon use of these agents for PTSD underscores the need for new research to further evaluate their efficacy and define their role.
To our knowledge, specific details of the use of SSRIs has not been examined in VA populations. Additional research and education are needed to further define and promote evidence-based prescribing practices for patients with PTSD.