Approximately 1.8 percent of the U.S. population is infected with the hepatitis C virus (HCV) (
1). HCV is transmitted via blood-to-blood contact. More than two-thirds of new infections result from injection drug use; thus individuals with substance disorders are at high risk of HCV (
1).
Although few studies have examined HCV infection among persons with serious mental illness, this population is also at high risk. One study prospectively tested 931 individuals with serious mental illness and found that 19.6 percent were HCV positive (
2,
3). The study also found that persons with serious mental illness may engage in increased risk behaviors; 45.9 percent had substance use disorders, 20.1 percent had injected drugs, and 14.5 percent had shared needles.
Current antiviral therapy includes pegylated or nonpegylated interferon alpha (IFN) and ribavirin combination therapy (
1). Most patients who receive IFN monotherapy or combination therapy report psychiatric side effects, and at least 20 percent report depressive symptoms (
4). Health care providers may be reluctant to treat HCV-infected adults with psychiatric or substance use disorders out of concern that the treatment may exacerbate symptoms or that patients may be noncompliant. Thus a majority of individuals with HCV may be regarded as ineligible for treatment (
5). However, there is no evidence to suggest that persons who have psychiatric or substance use disorders are more likely to have neuropsychiatric side effects or to be noncompliant than those without such disorders. Although samples have been small, several studies suggest that individuals with current psychiatric disorders, active injection drug use, or a history of alcohol abuse can be treated effectively and safely for HCV, in particular when treatment is comanaged with mental health providers or psychotropic medications (
6,
7,
8).
The National Institutes of Health (NIH) and the Veterans Healthcare Administration (VHA) have identified HCV as a major public health priority and have issued guidelines for identification and management (
1,
9). They recommend "wide availability of diagnostic tests," "establishment of screening tests for all groups at high risk," and the extension of treatment to special populations infected with HCV, such as those with psychiatric and substance use disorders (
1). However, few studies have evaluated current disease management practices, and the extent to which health care systems have implemented these recommendations is unclear.
The study reported here examined disease management trends in the Northwest VHA (Veterans Integrated Service Network 20 [VISN20]) during a period (1998 to 2003) marked by many changes in HCV treatment guidelines and policies. Although the 1997 NIH treatment guidelines listed depression and substance use disorders as contraindications to IFN therapy for HCV, the 2002 NIH guidelines removed these contraindications and instead encouraged increased treatment availability among these groups (
1). Also during this period, the VHA instituted several performance measures designed to guide HCV disease management. In 1998 the VHA secretary issued a letter recommending that all veterans be screened or tested for HCV, and in 1999 all VA facilities were mandated to implement universal screening for HCV risk factors and HCV antibody testing if risk factors were present. In 2000 the VHA introduced electronic clinical reminders in patients' medical records that prompted providers to screen and test for HCV. The clinical reminder included a standardized HCV risk factors questionnaire.
Local VHA facilities have likely varied in the timing of implementation of these guidelines. All VISN20 sites implemented the reminder system by 2001, and one site (Seattle) opted for universal HCV testing rather than screening. On the basis of a random sample of patient records, a performance measure summary for fiscal year 2002 estimated that 85 percent of all VISN20 patients were screened for HCV, and 65 percent who screened positive were tested. By fiscal year 2003 these numbers rose to 93 percent and 88 percent, respectively. No statistics for previous years are available. Although the study reported here does not specify the direct impact of these measures, it does highlight current performance trends in their historical context.
To conduct this retrospective database study, we first determined testing and infection rates among patients with schizophrenia and substance use disorders and compared the rates with those in a control group of patients without these diagnoses. We hypothesized that patients with these disorders would have higher infection rates and would be less likely to receive IFN therapy than the control group.
Methods
We collected data for all 293,445 patients treated between January 1998 and December 2003 at any facility in VISN20: eight medical centers and 17 outpatient clinics in Alaska, Washington, Oregon, and Idaho. Data came from the VISN20 CHIPS data warehouse, a collection of databases extracted from the electronic patient medical records of each facility. The Portland VHA Medical Center institutional review board approved data access for this project.
We collected data on demographic characteristics, psychiatric diagnoses, HCV laboratory results, and prescriptions. Laboratory results included tests performed between 1994 and 2003; data on IFN prescriptions were available only for 1998 to 2003. We excluded nonveterans who did not receive regular care from the VHA but who had records in the system.
We considered patients to have been tested for HCV if they had at least one HCV laboratory result in their record. HCV-positive patients had a positive HCV antibody test, a detectable HCV viral load by polymerase chain reaction, a positive HCV recombinant immunoblot assay (RIBA), or an identifiable HCV genotype. We classified patients with positive antibody tests but negative RIBA confirmation as false positives. Through patient prescription records, we established whether a patient had been given at least one prescription for antiviral therapy after the patient was identified as being HCV positive. Antiviral therapy included either monotherapy or combination therapy with pegylated or nonpegylated IFN.
We considered a patient to have a substance use disorder if the patient's record included a DSM-IV code for substance abuse or dependence (except nicotine dependence). A patient was considered to have schizophrenia or schizoaffective disorder if the patient's record included a DSM-IV code for either disorder. Our database did not allow for assessment of patient functioning, so a broader sample of patients with serious mental illness could not be defined.
We assigned patients to one of four mutually exclusive groups. The substance abuse group had a history of a substance use disorder but no history of schizophrenia or schizoaffective disorder. The schizophrenia group had a history of schizophrenia or schizoaffective disorder but no history of a substance use disorder. The co-occurring-disorders group had a history of both schizophrenia or schizoaffective disorder and a substance use disorder. The control group had no history of schizophrenia or schizoaffective disorder or a substance use disorder.
We downloaded data from the VISN20 data warehouse into a local database by using structured query language (SQL) queries and then organized and exported the data to SPSS 11.0 for analysis.
Results
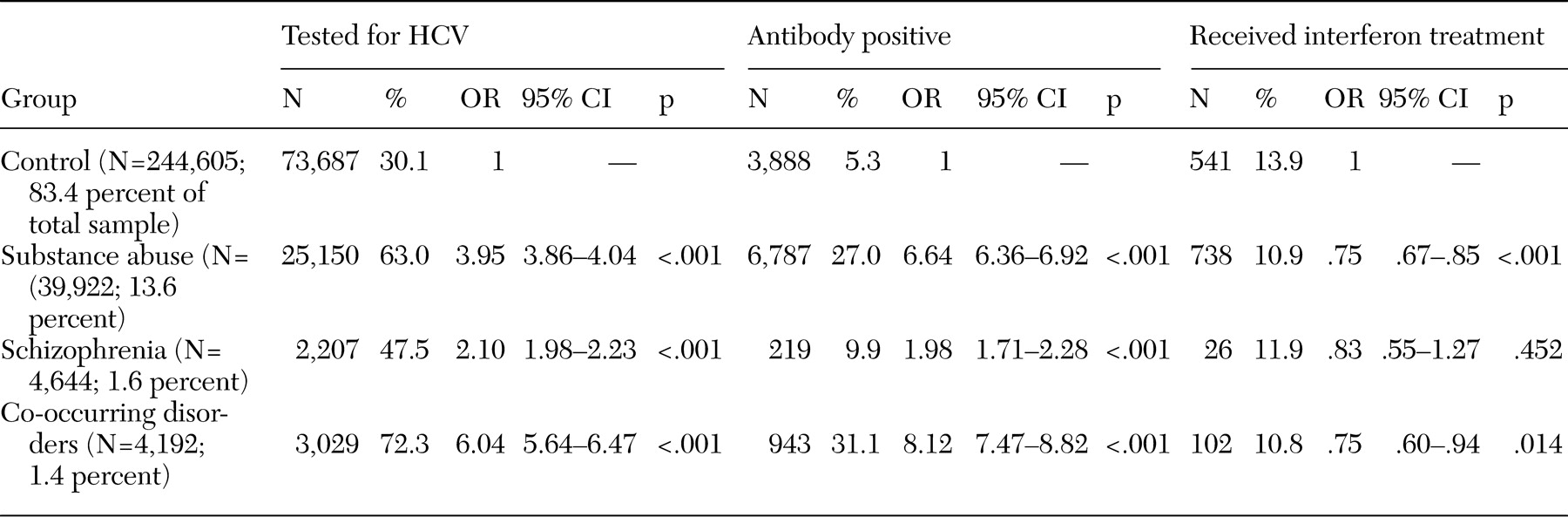
Of the total sample of 293,445 patients, 93.3 percent (N=273,786) were male. Of the 129,833 patients whose race was specified in the database, 86.3 percent (N=112,008) were Caucasian. The mean±SD age of the sample was 60.4±15.7 years. For the high-risk groups, 13.6 percent of the sample (N=39,922) were in the substance use disorders group, 1.6 percent (N=4,644) were in the schizophrenia group, and 1.4 percent (N=4,192) were in the co-occurring-disorders group. The control group constituted 83.4 percent (N=244,605) of the total sample.
In the total sample, 35.5 percent of patients received testing for HCV.
Table 1 summarizes our findings. Compared with the control group, the high-risk groups were all significantly more likely to receive HCV testing. HCV testing was administered to 63.0 percent of the substance use disorders group, 47.5 percent of the schizophrenia group, 72.3 percent of the co-occurring-disorders group, and 30.1 percent of the control group. Respectively, the high-risk groups were approximately four, two, and six times as likely to receive HCV testing compared with the control group.
Of patients tested for HCV in the total sample, 11.4 percent tested antibody positive. Compared with the control group, high-risk groups were all significantly more likely to test antibody positive; 27.0 percent of the substance use disorders group, 9.9 percent of the schizophrenia group, 31.1 percent of the co-occurring-disorders group, and 5.3 percent of the control group tested antibody positive. Respectively, the high-risk groups were approximately seven, two, and eight times as likely as the control group to have HCV infection. Because testing of patients with risk factors was mandatory and not all patients were tested, these infection rates may overestimate true infection rates.
In the total sample, which included patients who were and were not tested, 4.0 percent were antibody positive. In the study groups, 17.0 percent of the substance use disorders group, 4.7 percent of the schizophrenia group, 22.5 percent of the co-occurring-disorders group, and 1.6 percent of the control group tested antibody positive. These rates may underestimate true infection rates because of the likelihood of undetected cases among patients who were not tested.
Of all patients who tested positive for HCV, 11.9 percent received at least one prescription for IFN. Within groups, 10.9 percent of the substance use group, 11.9 percent of the schizophrenia group, 10.8 percent of the co-occurring disorders group, and 13.9 percent of the control group received IFN therapy. Compared with HCV-positive patients in the control group, patients who were HCV positive in the substance use group and the co-occurring-disorders group were significantly less likely to receive IFN. Patients who were HCV positive in the schizophrenia group were as likely to receive IFN as HCV-positive patients in the control group.
Discussion
This study examined HCV testing, infection, and treatment rates among 293,445 veterans who received medical care between 1998 and 2003 in facilities in the VISN20. Our hypothesis that patients in high-risk diagnostic groups would have higher rates of HCV infection was confirmed. Also, our hypothesis that patients in high-risk diagnostic groups would be less likely to receive IFN therapy was confirmed for patients with substance use disorders and with co-occurring disorders but not for patients with schizophrenia or schizoaffective disorder.
Previous studies have demonstrated higher HCV infection rates among veterans than in the general U.S. population (approximately 5.4 percent compared with 1.8 percent) (
10). Similarly, our study found that 11.4 percent of all tested veterans were antibody positive. This represented 4 percent of all veterans who sought medical care at the study facilities during the study period, regardless of whether or not they were tested. Those with a history of substance use disorder or co-occurring disorders were at higher risk and had a sevenfold and eightfold increase in the odds of HCV infection, respectively; of those tested, 27 percent and 31.1 percent were antibody positive, respectively.
Our findings are also consistent with those of previous studies of adults with serious mental illness. One study of 931 adults found that 19.6 tested antibody positive (
2). Unlike our study, this study tested prospectively and included a broader sample of patients with serious mental illness, some with psychiatric diagnoses other than schizophrenia and schizoaffective disorder. In our study, 22.2 percent of all tested veterans with schizophrenia or schizoaffective disorder (with or without a co-occurring substance use disorder) were antibody positive. This represented 13.2 percent of all patients with schizophrenia or schizoaffective disorder who sought care, regardless of whether or not they were tested. Even patients in the schizophrenia group with no history of a substance use disorder were twice as likely as those in the control group to have HCV infection, which suggests that a diagnosis of schizophrenia may be a risk factor independent of substance use disorder.
As predicted, patients with substance use disorders as well as those with co-occurring schizophrenia or schizoaffective disorder and a substance use disorder were significantly less likely to receive IFN therapy than patients in the control group. Because patients in the schizophrenia group received IFN therapy at a rate comparable to that of the control group, the trend for the co-occurring-disorders group may have been attributable to patients' history of substance abuse rather than to a diagnosis of schizophrenia. However, regardless of diagnostic status, few HCV-positive patients received IFN therapy—only 11.9 percent of all patients and 13.9 percent of control patients. Although differences between some groups were significant, overall, the magnitude of these differences was not substantial, suggesting that these high-risk groups have not been excluded from HCV therapy within VISN20.
The study had several limitations. As in other studies with retrospective database designs, it is likely that inconsistencies and errors in medical record documentation, missing data points, and preestablished database variables affected the scope of the findings. In particular, the design did not allow us to explore why patients had not been tested or treated for HCV, so it is unclear to what extent patient or provider variables may have affected testing and treatment rates. For example, veterans with schizophrenia or substance use disorders may have refused or been unavailable for HCV testing or treatment.
Additional limitations include the inability to confirm the accuracy of a patient's recorded diagnoses. VISN20 has instituted a performance measure whereby providers are annually required to complete a standard substance abuse screening questionnaire that automatically appears in each patient's record as a clinical reminder. Although providers in VISN20 are perhaps more likely to detect substance use disorders than providers in other institutions that do not have standard screening procedures, the brief screening is probably insufficient to detect all cases of substance use disorders. Thus this study may underestimate the actual frequency of substance use disorders in our sample. Future studies that use a thorough medical record review in selected diagnostic groups could better address these issues.
A strength of this study is its large sample. This study is the first study to assess HCV testing, infection, and treatment rates in a large health care organization and to compare particular diagnostic groups at high-risk of HCV infection. Although detection and treatment of HCV is a major public health priority, few studies have evaluated how health care networks manage this disease. This study made initial efforts to evaluate both disease and treatment trends within the largest health care network in the United States—the VHA.
Although our findings may not be generalizable to nonveteran populations, health care networks may still benefit from adopting VHA's practices. The VHA has implemented performance measures, universal screening for HCV risk factors, and mandatory testing of high-risk patients in order to increase HCV detection. It is likely that testing and treatment rates are higher in the VHA than in institutions that lack standard HCV screening procedures. VISN20 sites also house the Northwest Hepatitis C Resource Center, a nationally funded research center charged with developing HCV best practices. Thus VISN20 may be better able to provide complex care for its HCV patients than institutions without such resources. Compared with other regions, the Northwest may be less ethnically and racially diverse, and VISN20 serves a primarily male, middle-aged, and Caucasian population. At least one study found increased HCV infection rates among older adults and among males; after the analysis controlled for higher rates among metropolitan residents compared with nonmetropolitan residents, race was not found to be associated with infection (
2). VISN20 includes both urban and rural populations, but these populations may not reflect the demographic characteristics of populations in other regions of the United States.
Conclusions
In our retrospective database study, we found that patients with substance use disorders as well as patients with schizophrenia or schizoaffective disorder, regardless of whether they had co-occurring substance use disorders, had a significantly higher risk of HCV infection than patients who did not have these diagnoses. The proportion of patients with HCV who received antiviral therapy was significantly lower for veterans with substance use disorders and for veterans with co-occurring schizophrenia or schizoaffective disorder and substance use disorders than for veterans in the control group. However, the proportion of patients with HCV who received antiviral therapy was low for all groups. Reasons for the low treatment rates are unknown and must await further research. Future studies should also explore factors that influence testing rates and treatment outcomes in high-risk groups.
Acknowledgments
Dr. Hauser, who has additional affiliations with the departments of behavioral neuroscience and internal medicine and with the J.E.N.S. laboratory at Oregon Health and Science University, thanks these entities for supporting this work. This work was sponsored in part by the VA Merit Review Program through the Office of Research and Development and by a grant from the Stanley Medical Research Institute.