Although many neurotransmitter systems have been implicated in methamphetamine’s reinforcing effects, by far the most evidence points to the dopamine system (
1,
9). Most studies of the effects of repeated methamphetamine administration have concentrated on its neurotoxic effects on dopamine cells. In laboratory animals, methamphetamine induces profound and long lasting damage to dopamine cells (
10). Human postmortem and imaging studies in methamphetamine abusers have documented significant losses in dopamine transporters, which are structural elements of the dopamine terminals (
11–
13). In methamphetamine abusers, the loss of dopamine transporters has been associated with motor and cognitive impairment (
13) but has not been linked to the mechanisms underlying methamphetamine addiction. Moreover, little is known about the role of dopamine in the loss of control and compulsive drug intake seen in methamphetamine-addicted subjects.
Imaging studies have shown that a common abnormality in drug-addicted subjects, including alcoholics, cocaine abusers, and heroin abusers, is a lower than normal level of dopamine D
2 receptor availability (
14–
17). Moreover, we have shown an association in cocaine abusers between striatal dopamine D
2 receptor densities and metabolic rates in the orbitofrontal cortex (
18). Since disruption of the orbitofrontal cortex is associated with obsessive and compulsive behaviors (
19,
20), we hypothesized that dopamine dysregulation of the orbitofrontal cortex underlies compulsive drug intake in cocaine-addicted subjects (
21,
22). Here we evaluated the question of whether a similar disruption could underlie methamphetamine addiction.
Results
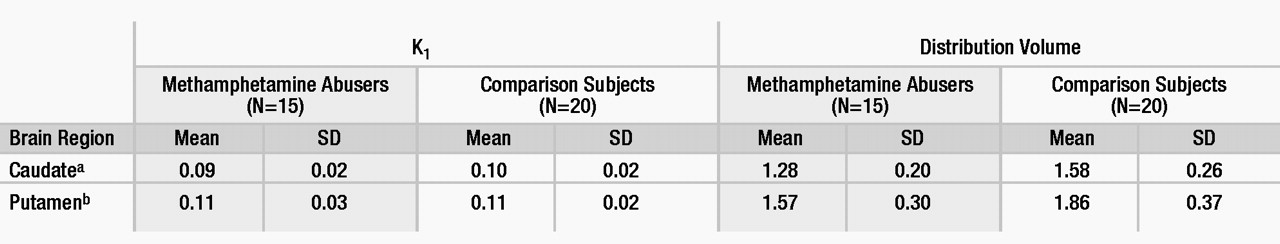
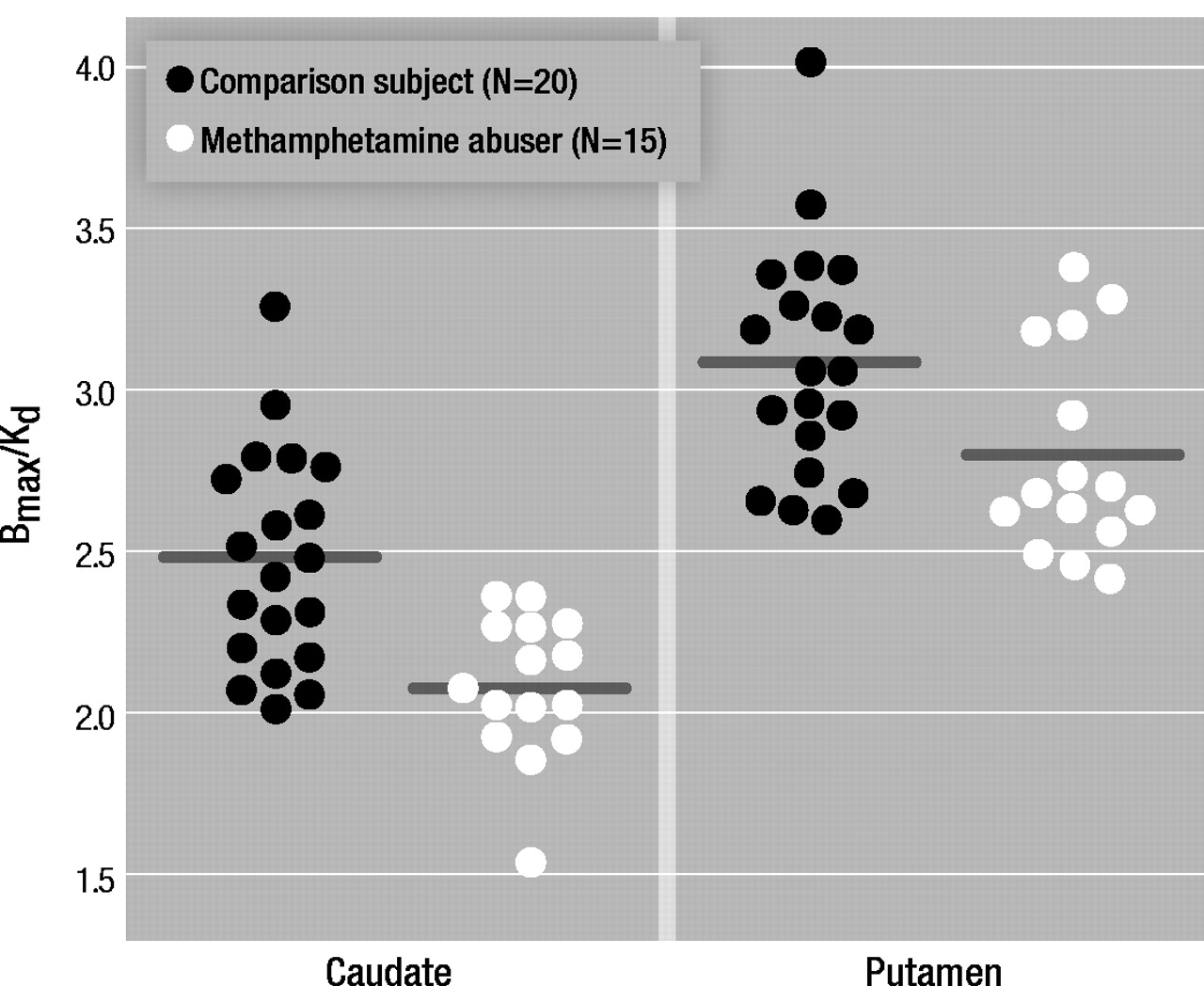
The transport rate parameters of [11C]raclopride from plasma to brain (K1) for the striatum or for the cerebellum did not differ between the methamphetamine abusers and the comparison subjects. The distribution volume in the caudate and the putamen, but not in the cerebellum, was significantly lower in the methamphetamine abusers than in the comparison subjects (Table 1). The estimates of dopamine D2 receptor availability (Bmax/Kd) were significantly lower in the methamphetamine abusers than in the comparison subjects in both the caudate and the putamen (Figure 1). Although the differences in Bmax/ Kd between the groups appeared larger in the caudate than in the putamen (a difference of 16% in the caudate versus 10% in the putamen), these differences between the regions were not significant, according to results of a one-factor (comparison subjects versus methamphetamine abusers), repeated measures (caudate versus putamen) ANOVA (F=0.87, df=1, 33, p=0.36). No differences in Bmax/Kd in the caudate and the putamen were found between the 12 methamphetamine abusers who had last used methamphetamine within 5 months of the study and the three who had not used methamphetamine for more than 11 months (caudate: mean Bmax/Kd=2.08, SD=0.25, and 2.09, SD=0.17, respectively [F=0.001, df=1, 14, p=0.97]; putamen: mean Bmax/Kd=2.81, SD=0.53, and 2.80, SD=0.40, respectively [F=0.002, df=1, 14, p=0.97]).
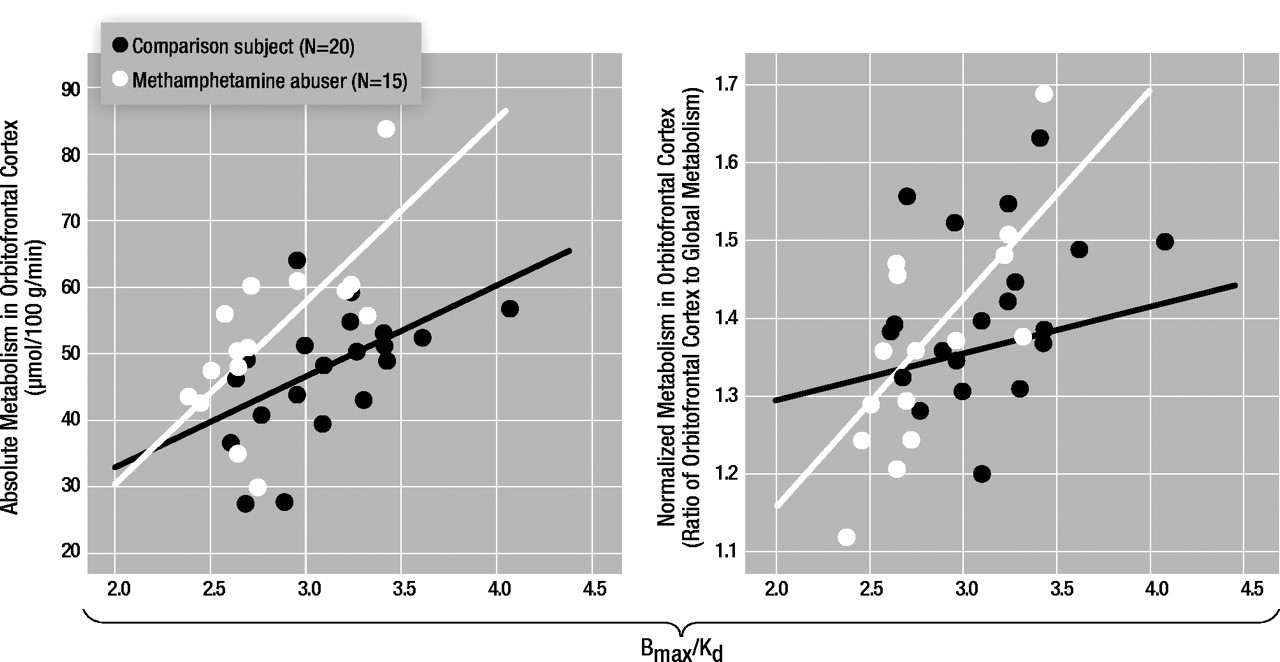
In the methamphetamine abusers, the dopamine D2 receptor availability measures in the putamen were correlated with metabolism in the orbitofrontal cortex (r=0.69, df=14, p<0.005) but not with metabolism in the caudate (r=0.40, df=14, p=0.15), putamen (r=0.39, df=14, p=0.15), temporal cortex (r=0.34 df=14, p=0.22), or cerebellum (r=0.36, df=14, p=0.19). Similar results were observed in the comparison subjects, for whom a significant correlation was found only between dopamine D2 receptor availability in the putamen and metabolism in orbitofrontal cortex (r=0.52, df=19, p<0.02). None of the correlations with dopamine D2 receptors in the caudate in the methamphetamine abusers or the comparison subjects were significant (data not shown). There were no differences in metabolism in the orbitofrontal cortex between the comparison subjects (mean=48 μmol/100 g per minute, SD=9) and the abusers (mean=52 μmol/100 g per minute, SD=13), but D2 receptor availability was lower in methamphetamine abusers. Therefore, the regression slopes appear to show that for a given estimate of dopamine D2 receptor availability, the absolute metabolism in the orbitofrontal cortex is higher in the methamphetamine abusers than in the comparison subjects (Figure 2). However, a covariate analysis to test for differences between the groups in orbitofrontal metabolism conditional on the D2 measures was not significant.
For the methamphetamine abusers, the correlations between the dopamine D2 receptor measures and the normalized metabolic measures (measure for the region of interest divided by the measure for the whole brain) showed results similar to those for the absolute metabolic measures: the only significant correlation was between D2 receptor availability in the putamen and metabolism in the orbitofrontal cortex (r=0.74, df=14, p<0.002) (Figure 3). In the comparison subjects, none of the correlations with the normalized metabolic measures was significant, including the correlation between the measure of D2 receptor availability in the putamen and metabolism in the orbitofrontal cortex (r=0.31, df=19, p=0.18).
Discussion
Imaging and postmortem studies have documented in methamphetamine abusers markedly lower levels of dopamine transporters, which serve as presynaptic markers for the dopamine terminal (
11–
13). However, to our knowledge, this is the first PET study to document lower levels of dopamine D
2 receptors in methamphetamine abusers. PET measures of dopamine D
2 receptors mostly reflect the level of postsynaptic receptors (
28). Thus, these findings provide evidence that methamphetamine also affects postsynaptic dopamine elements, which in the striatum most likely reflect effects of methamphetamine on intrinsic γ-aminobutyric acid cells. These findings corroborate the few preclinical studies showing that methamphetamine, in addition to causing changes in presynaptic dopamine markers, also reduces postsynaptic dopamine D
2 (
29,
30) and D
1 receptors (
31).
Lower levels of D
2 receptor availability in methamphetamine abusers could reflect receptor down-regulation from exposure to a higher extracellular dopamine concentration secondary to methamphetamine’s acute pharmacological effects as well as methamphetamine-induced losses of dopamine transporters (
11). Even though studies have documented decreased striatal dopamine concentration with methamphetamine administration (
11,
32), the concomitant dopamine transporter losses could still result in enhanced extracellular dopamine, as shown in dopamine-transporter knockout mice (
33). Alternatively, the low levels of D
2 receptors could have preceded methamphetamine use and may have predisposed these subjects to drug use. In support of this possibility is a study showing that, in non-drug-abusing comparison subjects, striatal D
2 receptor levels predicted responses to psychostimulant administration (
34). Subjects with low D
2 receptor levels experienced a “pleasurable” response, whereas subjects with high receptor levels experienced an “unpleasant” response. These findings led us to speculate that D
2 receptors, by modulating pleasant versus unpleasant drug responses, may be a variable that contributes to drug abuse and addiction. However, in the study reported here, it was not possible to determine if the lower levels of dopamine D
2 receptors preceded the use of methamphetamine use or reflected chronic use and, if the lower levels resulted from chronic use, whether they recover with detoxification. Although in this study we were unable to detect differences in D
2 receptors between the 12 methamphetamine abusers tested within 5 months of last methamphetamine use and the three tested after 11 months of detoxification, the size of the study group was too small to determine if recovery of D
2 receptors occurs with detoxification. Also, since [
11C]raclopride is sensitive to endogenous dopamine, we cannot rule out the possibility that the lower levels of D
2 receptor availability could reflect competition of [
11C]raclopride binding with dopamine (
35).
Reductions in D
2 receptors have been reported in other drug abusers, including cocaine abusers (
14,
18,
36), alcoholics (
15,
37), and heroin abusers (
17), suggesting that reductions in D
2 receptors are not specific to any type of drug addiction but may underlie a common abnormality in addicted states and/or a common predisposing factor. Moreover, we recently demonstrated a lower level of D
2 receptors in pathologically obese subjects, who share with drug-addicted subjects the compulsive administration of the reinforcer, which for obese subjects is not a drug but food (
38).
The association between metabolic activity in the orbitofrontal cortex and measures of D
2 receptors could reflect dopamine-mediated striatal regulation of orbitofrontal activity by means of striato-thalamo-cortical pathways (
39). The orbitofrontal cortex receives projections both from the nucleus accumbens (
40), which is the region in the striatum that is traditionally associated with the reinforcing effects of drugs of abuse (
41), and from the ventral tegmental area, which is the main dopamine projection to the nucleus accumbens (
42). However, the orbitofrontal cortex also sends projections to the nucleus accumbens (
39), so we cannot rule out the possibility that the association reflects orbitofrontal regulation of dopamine striatal activity. The reciprocal neuroanatomical connections between the orbitofrontal cortex and the nucleus accumbens make the orbitofrontal cortex a direct target for the effects of drugs of abuse and a region that could modulate these responses. Because of the limited spatial resolution of the PET camera, dopamine D
2 receptor measures were quantified in the putamen (one cannot accurately measure receptor availability in nucleus accumbens). Future studies done with PET cameras with better spatial resolution and sensitivity are required to specifically evaluate the association between activity in the orbitofrontal cortex and measures of D
2 receptors in the nucleus accumbens.
The relationship between metabolic activity in the orbitofrontal cortex and the availability of D2 receptors was significant both in the methamphetamine abusers and the comparison subjects. However, the association between D2 receptors and the normalized metabolic measures in the orbitofrontal cortex was significant in the abusers but not in the comparison subjects. Since normalized measures are more sensitive to regional changes than absolute measures, this association could reflect a higher sensitivity of the orbitofrontal cortex to dopamine modulation in methamphetamine abusers than in comparison subjects. However, further studies are required to determine if there is enhanced sensitivity of the orbitofrontal cortex to dopamine modulation and/or enhanced striatal dopamine regulation by the orbitofrontal cortex in drug-addicted subjects, compared with non-drug-addicted subjects.
Dopamine modulation of the orbitofrontal cortex could underlie addictive behaviors in several ways. First, the orbitofrontal cortex is involved in the regulation of “drive” (
43), and thus enhanced activation secondary to drug-induced dopamine stimulation could result in an intense motivation to self-administer methamphetamine in the addicted subjects. Moreover, because the orbitofrontal cortex processes information about the rewarding properties of stimuli (
44), its disruption could account for the enhanced salience of drug-related stimuli. Second, the orbitofrontal cortex has been implicated in the occurrence of compulsive behaviors (
19,
20), and thus one could postulate that its inappropriate activation could induce compulsive drug administration in methamphetamine abusers. In laboratory animals, damage to the orbitofrontal cortex results in per severation and resistance to extinction of reward-associated behaviors (
45,
46). These findings are reminiscent of the reports of drug addicts who claim that once they start taking a drug of abuse they cannot stop even when the drug is no longer pleasurable. Third, the orbitofrontal cortex is involved with learning stimulus-reinforcement associations (
47) and with conditioned responses (
48) and could therefore participate in cues or drug-induced craving. Laboratory animals exposed to an environment where they had received a drug of abuse show orbitofrontal activation (
49), and lesions of the orbitofrontal cortex interfere with drug-induced conditioned place preference (
50). These findings are relevant because drug-induced conditioned responses have been implicated in the craving elicited in humans by drug-related stimuli (
51), which is one of the factors that contributes to relapse in drug abusers (
52). Moreover, activation of the orbitofrontal cortex has been shown in drug abusers during craving elicited by a drug (
53), by viewing a video of drug paraphernalia (
54), and by recalling previous drug experiences (
55).
Limitations for this study are those inherent in clinical research in drug abuse populations, including inaccuracies in clinical histories and histories of drug use by the subjects, as well as confounds from differences between the groups investigated (e.g., differences in levels of consumption of nicotine or alcohol). Thus, in this study, we were unable to completely rule out the influence of comorbid factors. In interpreting these results, one also needs to consider the importance of other brain regions, other dopamine receptor subtypes, and other neurotransmitter systems in the modulation of the orbitofrontal cortex and in methamphetamine addiction.
This study shows lower levels of dopamine D2 receptor availability in methamphetamine abusers than in non-drug-abusing comparison subjects, providing evidence for the involvement of postsynaptic cells in the effects of methamphetamine on dopamine neurotransmission. The significant association between dopamine D2 receptors in the putamen and activity in the orbitofrontal cortex, a brain region involved with compulsive behaviors, suggests that dysregulation of the orbitofrontal cortex may be one of the mechanisms by which disruption of dopamine activity in methamphetamine abusers could lead to compulsive drug-taking behavior.