Substantial recent interest has been shown in the issue of comorbidity between major depression (MD) and anxiety disorders (
Maser and Cloninger, 1990). In this report, we take a genetic epidemiological approach to this problem. In particular, we are interested in disentangling three different sources of comorbidity between MD and anxiety disorders:
(a) Genetic factors that contribute to the liability to both MD and anxiety disorders. In genetic par-lance, this is termed pleiotropy—a single gene or set of genes influencing more than one disorder or set of traits.
(b) Familial environmental factors, such as parental rearing style (
Parker, 1979,
1981), that increase risk for both MD and anxiety disorders.
(c) Individual specific environmental factors (i.e. unshared by a twin with her co-twin), such as most stressful life events (SLEs) of adulthood, which influence the liability to both MD and anxiety disorders.
Although epidemiological samples are critical for documenting the magnitude of comorbidity, free of the effects of Berkson’s bias (
Berkson, 1946), genetically informative samples are required to clarify the role of genetic versus environmental factors in the aetiology of comorbidity. Although family studies can discriminate the impact of individual specific environmental factors from those of genes and the family environment, they are unable to make the important distinction between these latter two potential causes of comorbidity. For this distinction, twin or adoption designs are needed.
The first multivariate genetic analysis of anxiety and depression of which I am aware was performed on self-report symptoms of anxiety and depression in 3798 pairs of twins from the volunteer Australian National Health and Medical Research Council Twin Registry (
Kendler et al, 1987). Thirteen symptoms from the anxiety and depression sub-scales of the Delusions-Symptoms-States Inventory (
Bedford et al, 1976) were examined. The best-fitting multivariate genetic model suggested that symptoms of anxiety and depression were moderately influenced by genetic factors. However, these genes appeared to act largely in a non-specific way to influence the overall level of anxiety and depression. No evidence could be found for genes that specifically influenced liability to depressive symptoms without also affecting liability to anxiety. By contrast, environmental factors not shared by twins seemed to have specific effects: that is, some environmental experiences appeared to be either primarily anxiogenic or depressogenic. These results replicated across the sexes.
Since these results were based solely on self-report symptoms assessed by mailed questionnaires, their relevance to the clinical syndromes of anxiety and depression is uncertain. Therefore, I revisited this question when we had completed our first wave of personal interviews with both members of 1033 pairs of female-female twins from the population-based Virginia Twin Registry (
Kendler et al, 1992a). Using a modified Structured Clinical Interview for DSM-III-R (SCID;
Spitzer et al, 1987), we assessed lifetime history for MD and several anxiety disorders.
The first bivariate twin analysis focused on the lifetime risk for MD and generalised anxiety disorder (GAD) (
Kendler et al, 1992b). We found strong evidence for comorbidity in this epidemiological sample of women. If GAD was assessed without a diagnostic hierarchy, then the odds ratio (OR) for GAD given MD was 8.9 and 8.5 using, respectively, a one-month minimum duration for GAD (as in DSM-III;
American Psychiatric Association, 1980), or a six-month minimum duration (as in DSM-III-R;
APA, 1987). If GAD was assessed with a diagnostic hierarchy (so that GAD that occurred solely during a MD episode was not diagnosed), then the OR declined to 3.9 for one-month GAD. Six-month GAD with diagnostic hierarchy was not examined because of its rarity.
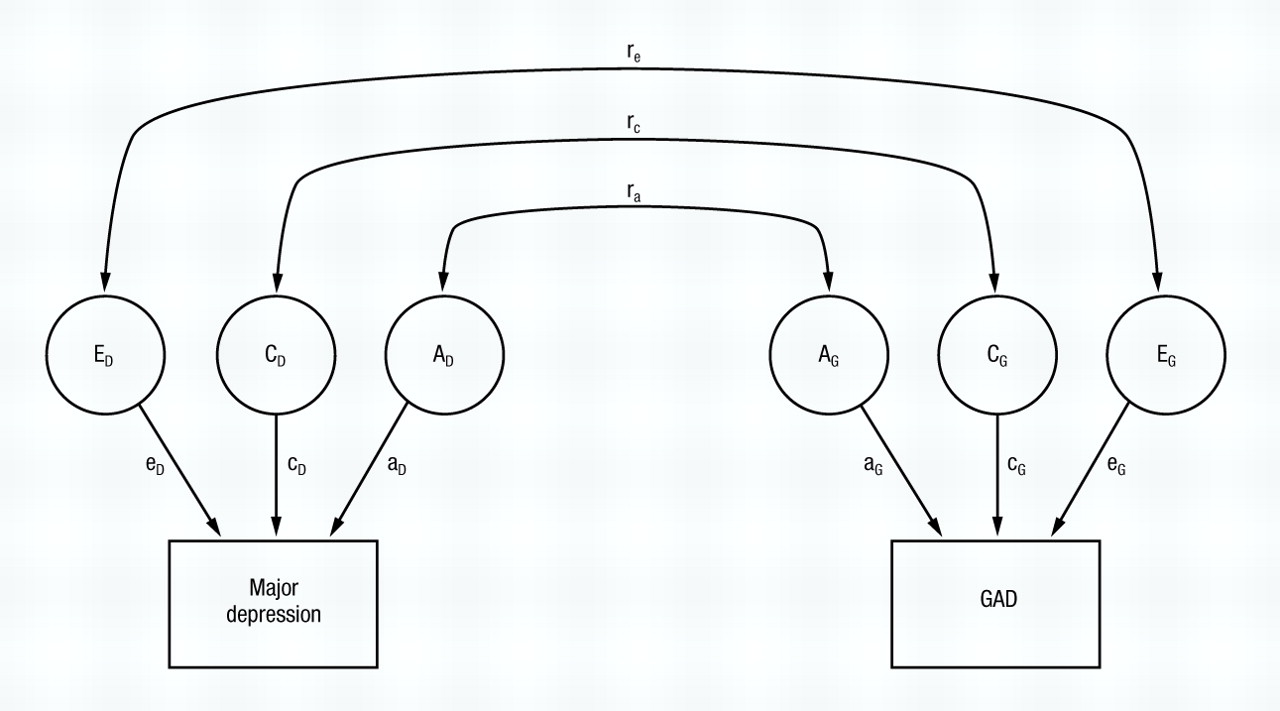
We then proceeded to fit formal bivariate twin models. The full model is pictured in Fig. 1. For all three definitions of GAD, the results were the same: the genetic correlation between MD and GAD could not be distinguished from unity. These results suggested that all the genes that influenced the liability to MD also influenced, in a similar way, the liability to GAD, and vice versa. As we had found in the past when examining the disorders one at a time, there was no evidence in these bivariate analyses for an aetiological role for family or common environment in MD (
Kendler et al, 1992a) or GAD (
Kendler et al, 1992c). (This conclusion does not mean that families are necessarily unimportant as potential causes of later psychopathology. However, these results do suggest, consistent with a range of other findings (
Plomin, 1994), that if families influence the later risk for MD or GAD, the impact tends to be different for different siblings.) However, the correlation in unique environmental factors in the aetiology of MD and GAD was significantly different from both unity and zero, ranging from +0.51 with one-month GAD without hierarchy, to +0.19 with one-month GAD with hierarchy. These results suggest that, although some environmental factors are both anxiogenic and depressogenic, other environmental factors are relatively specific in their impact on the liability either to MD or GAD.
The second set of bivariate twin analyses we conducted were on major depression and phobias (
Kendler et al, 1993). Using a modified version of the Diagnostic Interview Schedule (DIS;
Robins and Helzer, 1985) interview, we had assessed, in our personal interview with the twins, four classes of phobias: agoraphobia, social phobia, animal phobia, and situational phobia (largely claustrophobia and acrophobia) (
Kendler et al, 1992d). The OR for a lifetime diagnosis of MD given any phobia was relatively modest (2.3). For the specific phobias, it ranged from 4.9 for agoraphobia to 1.6 for animal phobia. The same bivariate twin models fitted to MD and GAD were fitted to MD and phobias, with very different results. Genetic correlations between MD and the various phobias were all quite low and significantly less than unity, ranging from +0.21 for any phobia to +0.38 for agoraphobia. Clearly the cause of comorbidity with MD differs widely across anxiety disorders. Although GAD and MD appear to share the same genetic risk factors, the genes that influence risk to phobias are only weakly related to those that influence the risk for MD.
In this paper, we re-examine the relationship between the genetic risk factors for MD and GAD. Since our initial report in the female-female Virginia twin sample, follow-up telephone inter-views have been completed with over 90% of the sample. We attempt, then, to replicate our previous finding by analysing, using a bivariate twin model, one-year prevalence for MD and GAD as assessed at this follow-up interview.
Method
We have outlined in detail elsewhere both the nature of this sample and our general approach to the analysis of population-based twin data (
Kendler et al, 1992a;
Kendler, 1993). In brief, this sample of Caucasian female-female twins was obtained from the population-based Virginia Twin Registry, which in turn was formed from a systematic review of birth certificates in the Commonwealth of Virginia. Twins were eligible to participate if both members of the pair had previously responded to a mailed questionnaire. In the first wave of personal inter-views, we succeeded in evaluating 2163 (92%) of the 2352 eligible individual twins. Zygosity was determined blindly by standard questions (
Eaves et al, 1989), photographs, and when necessary, DNA (
Spence et al, 1988).
Attempts were made to recontact all originally interviewed twins a minimum of one year after their first (time-1) interview. We succeeded in completing second (time-2) personal interviews in 2002 (93%) of them, including both members of 93 pairs of known zygosity, of which 547 were monozygotic (MZ) and 390 dizygotic (DZ). Unlike the time-1 interview (of which 89% were done in person), nearly all of the time-2 interviews (99%) were completed by phone. The average number of months (±s.d.) between the time-1 and time-2 interviews was 17.0±3.7. The time-2 interviews were conducted by trained mental health professionals (mostly social workers) blind to knowledge of the psychopathological status of the co-twin.
The time-2 interviews enquired of all respondents whether, in the last year, they had experienced any of 20 individual psychiatric symptoms, including all of the DSM-III-R criteria for major depression, and two screening questions each for GAD and panic disorder. For GAD, the two screening questions were:
(1) In the last year, have you had a time lasting at least five days when you felt anxious, nervous, or worried most of the time?
(2) In the last year, have you had a time lasting at least five days when most of the time your muscles felt tense, or you felt jumpy or shaky inside?
Note that the final phrase of the first screening question is very similar to that used in the SCID (
Spitzer et al, 1987) to assess lifetime GAD. The second question was added to assess the somatic manifestations of general anxiety. Any twin endorsing either of these two questions was then asked 18 items reflecting the individual D (or symptomatic) criteria for GAD in DSM-III-R. The diagnosis of MD was made by applying, by computer algorithm, all the relevant DSM-III-R criteria for a major depressive episode. We have previously shown that we can assess both GAD and MD in this sample with high interrater reliability (
Kendler et al, 1992a,
c).
As with our first interview, in the second follow-up interview we did not formally assess whether the anxiety or worry affected two or more life circumstances. Thus, our interpretation of this criterion for GAD is similar to DSM-III, but probably somewhat broader than that of DSM-III-R: In our analyses we used two definitions of GAD, both of which followed DSM-III-R in requiring six or more symptoms endorsed from the 18 in criterion D. The first definition reduced the required minimum duration of illness from six months (as in DSM-III-R) to one month (as in DSM-III) and eliminated the criterion C (“The disturbance does not occur only during the course of a mood disorder or a psychotic disorder”). This will be called “one-month GAD without hierarchy.” The second definition, called “one-month GAD with hierarchy,” differed from the first definition only in the application of criterion C; that is, if a twin met criteria for GAD only during times when criteria were also met for MD, that twin would receive the diagnosis of GAD by the first, but not by the second set of criteria. In our study of one-year prevalence, the prevalence of GAD requiring a minimum six-month duration of illness (1.4%) was too low to permit a meaningful analysis.
Statistical analysis
As outlined in detail elsewhere (
Kendler, 1993), twin studies are able to discriminate genetic from familial-environmental effects because MZ twins share all their genes in common, while DZ twins, on average, share half their genes identical by descent. Assuming that the environmental experiences that influence the trait in question are equally correlated in the two twin types (i.e. the equal environment assumption), excess similarity of MZ over DZ twins can be ascribed to genetic factors. Correlations in DZ twins that are greater than half that of MZ twins (the pattern predicted if only genetic factors were operative) can then be ascribed to familial environmental influences.
In univariate twin analysis, the goal is to decompose the variance in liability into its genetic and environmental components: In bivariate analysis, the main goal is to decompose the covariance between two disorders (here MD and GAD) into that due to genes, common environment, and unique environment. The full model is illustrated in Fig. 1. Basically, bivariate analysis subdivides the observed (or phenotypic) correlation in liability between two disorders into three parts: (a) that due to the same additive genes predisposing to both disorders (the additive genetic correlation or ra); (b) that due to the same common or familial environmental factors predisposing to both disorders (the common environmental correlation or rc); and (c) that due to the same unique environmental risk factors predisposing to both disorders (the unique environmental correlation or re).
Although univariate genetic analysis is conducted using MZ and DZ twin correlations for a single variable, bivariate analysis examines three kinds of correlations: within-variable cross-twin, within-twin cross-variable, and cross-twin cross-variable. See
Kendler et al (1992b) for further discussion of bivariate twin models.
Correlations are estimated by the computer program PRELIS (
Joreskog and Sorbom, 1988), assuming a liability threshold model. Model-fitting to these correlations of liability was performed by the computer program Mx (
Neale, 1991) using asymptotic weighted least squares (
Neale and Cardon, 1992). Nested models were then fitted, with the best-fitting model chosen using Akaike’s information criterion (AIC;
Akaike, 1987). The AIC, which equals χ
2 minus twice the number of degrees of freedom, reflects both the goodness of fit and parsimony of the model. The goal was to produce the model with the most negative value for AIC. Neither dominance genetic variance nor age effects were included in our analyses because our previous univariate analyses of MD (
Kendler et al, 1992a) and GAD (
Kendler et al, 1992c) failed to demonstrate consistently their importance for either disorder.
To assess possible violations of the “equal environment assumption,” twins were asked at time-1 the frequency with which they saw or had contact with their co-twins, with responses ranging from every day to once a year or less. Controlling for zygosity, we then examined by logistic regression whether frequency of contact predicted twin similarity for episodes of MD or GAD in the subsequent year.
Results
Association between GAD and MD and the equal environment assumption
Of the 1874 individuals from pairs of known zygosity in which both twins were assessed, 179 (9.6%) met criteria for MD in the last year. Criteria for one-month GAD without and with hierarchy were met by 144 (7.7%) and 80 (4.3%) twins, respectively. The association between MD and one-month GAD without hierarchy was very strong (OR=26.1, 95% CI 17.6–38.7; tetrachoric=+0.79). The association between MD and one-month GAD without hierarchy, although weaker, was also quite substantial (OR=4.0, 95% CI 2.4–6.6; tetrachoric=+0.35).
Controlling for zygosity, the frequency of contact at the time-1 interview did not predict twin concordance for subsequent episodes of MD (χ2=0.5, d.f.=1, NS) or GAD (χ2=1.43, d.f.=1, NS).
Model fitting: one-month GAD without hierarchy
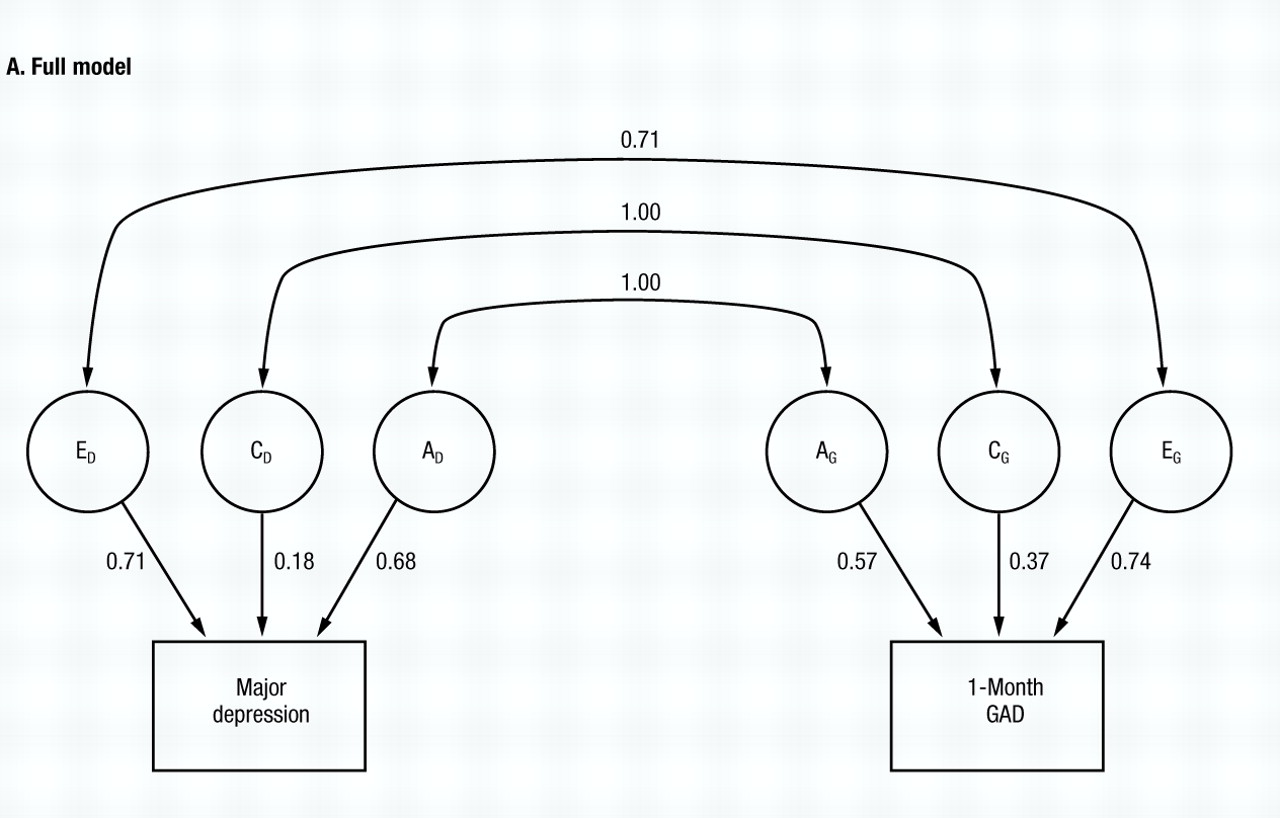
The full model (model I, Table 1) fits relatively well (χ2=10.39, d.f.=5, NS) and the parameter estimates are seen in Fig. 2A. Both the additive genetic (rc) and common environmental correlations (re) are estimated at unity, while the specific environmental correlation (re) is estimated at +0.71. However, the proportion of variance in liability to the two disorders accounted for by common environment is relatively modest (3% for MD and 13% for GAD). In models II, III and IV, we set to zero each of the correlations in turn. AIC becomes more positive when either ra (model III) or re is set to zero (model IV). However, the fit of the model is hardly altered when rc is set to zero (model II), and the AIC of model II is substantially more negative than that for model I.
Working from model II, then, we set each of the two remaining correlations to unity in turn. Although the fit deteriorates badly when re is set to one (model VI), the fit is unaltered and the AIC becomes more negative when ra is set at one (model V).
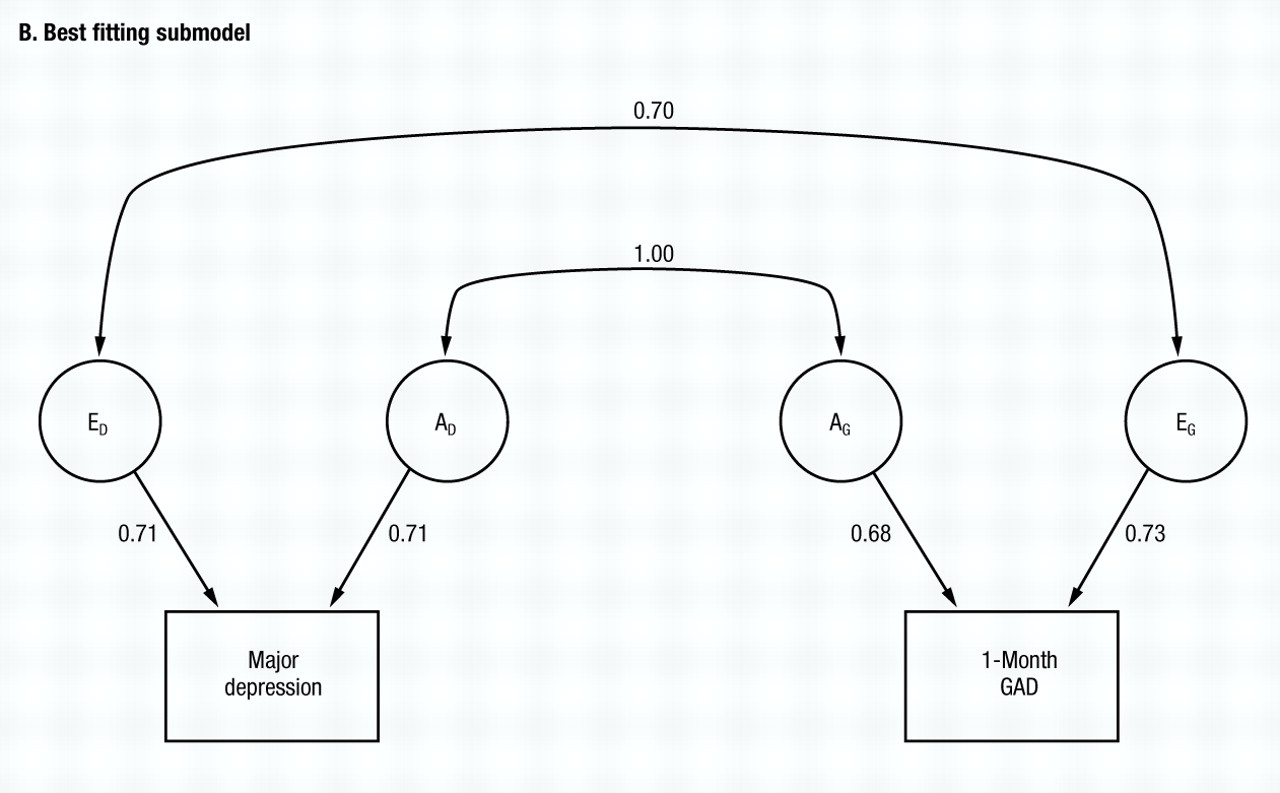
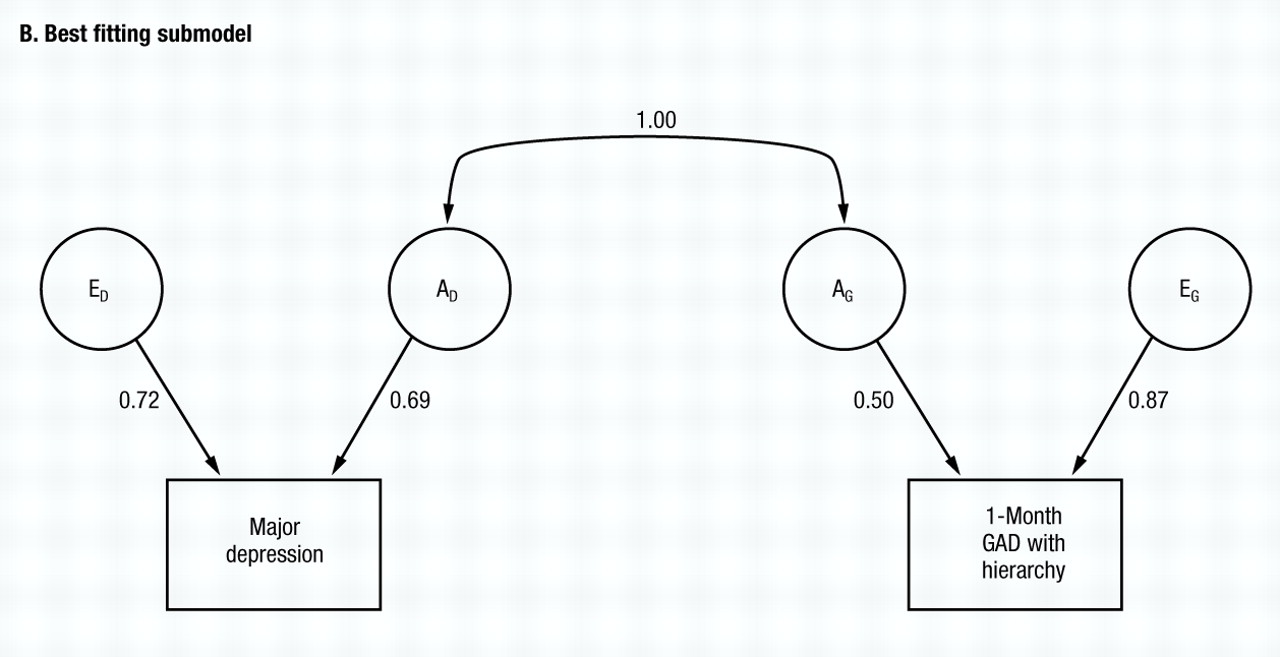
Working from model V, we then try to eliminate the other parameters from the twin model. C can be eliminated from both MD (model VII) and subsequently from GAD (model VIII), with the AIC taking on a yet larger negative value. Finally, we try setting re to zero (model IX) with a marked deterioration in fit. No further improvements could be made, making model VIII the best-fitting model. Parameter estimates of this model are seen in Fig. 2B, with re and ra estimated at +0.70 and 1.00, respectively. The phenotypic correlation between GAD and MD can now be decomposed according to the best-fitting model, with 57% resulting from genes that predispose to both disorders, and 43% resulting from individual specific environmental factors that increase risk for both disorders.
Model fitting: one-month GAD with hierarchy
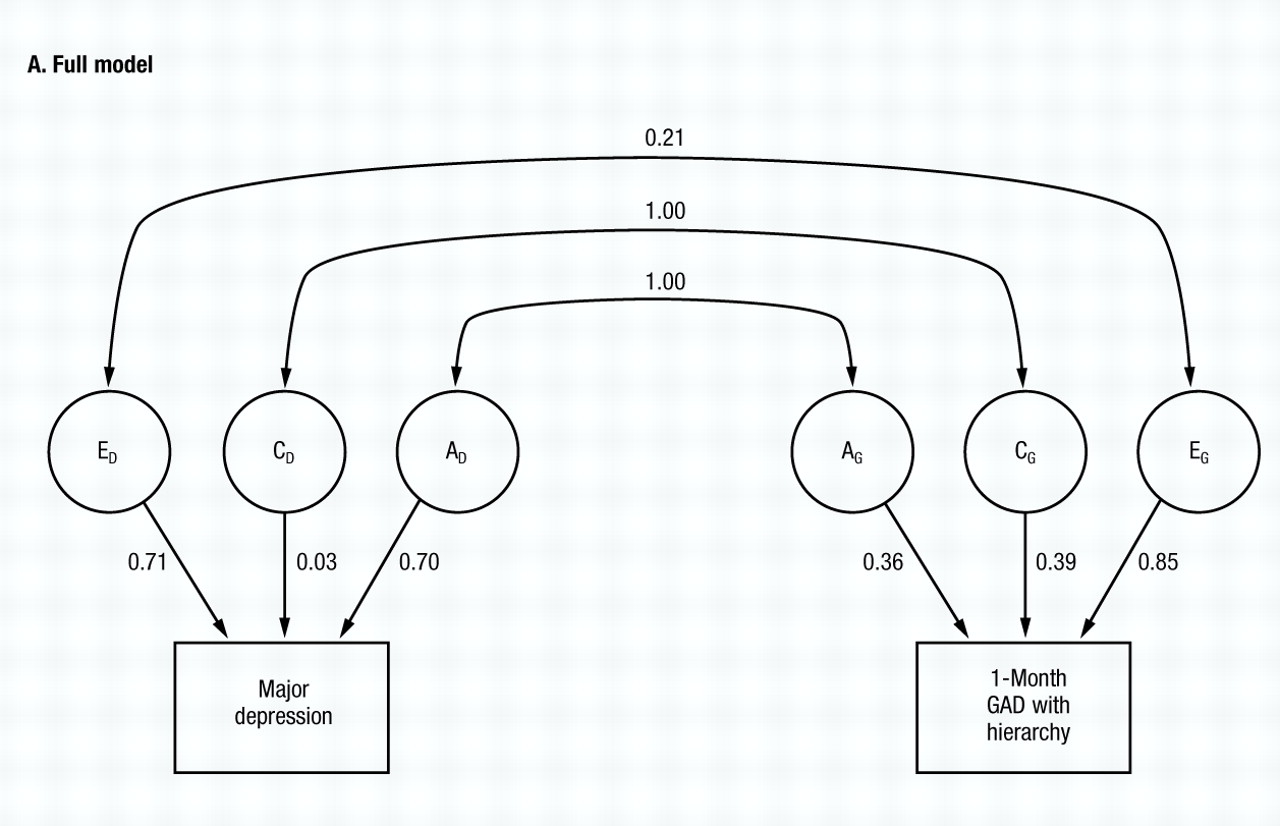
The full model (model I, Table 2) fits quite well (χ2=6.25, d.f.=5, NS) and (Fig. 3A) estimates both ra and rc at unity. By contrast, re is estimated at +0.21.
The common environmental component for MD is quite small, but for GAD is substantial. Despite some differences from the results with GAD without hierarchy, the results obtained in the model fitting are, nonetheless, rather similar. Among models II–IV, the best fit is obtained after setting re to zero (model II) In models V and VI, the best value of AIC is obtained when ra is set to unity (model V), although the AIC also improved marginally when re was set to unity in model VI. The common environment for both MD and GAD can be eliminated (models VII and VIII) with further improvements in the AIC. From model VIII, we then attempted to set re to either zero (model IX) or again to unity (model X). The former produced a substantial improvement and the latter a large deterioration in the AIC. Thus, model IX provided the best overall fit and no further improvement was possible. The parameter estimates of this very simple model are seen in Fig. 3B. Although the genetic correlation between MD and GAD is estimated at unity, the environmental correlation is estimated at zero.
Comment
Our goal in this paper was to attempt to replicate three major results from previous analyses from our epidemiological cohort of female-female twin pairs, obtained using the Virginia Twin Registry (
Kendler et al, 1992b): (a) MD and GAD demonstrated high levels of comorbidity; (b) the genetic factors influencing the liability to these two disorders are highly correlated, if not identical; and (c) the environmental risk factors for these two disorders are at least partially independent of one another.
Magnitude of comorbidity
Examining one-year prevalence, we replicated our previous results with lifetime prevalence, again finding high levels of comorbidity between MD and GAD. Between MD and one-month GAD without hierarchy, we found stronger associations in our one-year versus lifetime data, although this difference was considerably more marked using ORs (26.2 v. 8.9) than with tetrachoric correlations (+0.79 v. +0.68). By contrast, using one-month GAD with hierarchy, the association was very similar in the one-year versus lifetime data using ORs (4.0 v. 3.9). Using tetrachoric correlations, the one-year data yielded modestly weaker evidence for comorbidity than did the lifetime data (+0.35 v. +0.45, respectively).
Causes of comorbidity
The most important result of these analyses is that we replicated, with new data on one-year pre-valence for a year after the completion of our previous interview, our earlier analyses based on lifetime prevalence (
Kendler et al, 1992b), which found that the genetic correlation between MD and GAD did not significantly differ from unity. These results, which were seen both when GAD was diagnosed with and without a diagnostic hierarchy, provide further support for our previously articulated hypothesis (
Kendler et al, 1987) that genetic factors are relatively non-specific in their impact on symptoms of depression and generalised anxiety.
Our findings are also consistent with previous results in another potentially significant way. Previously, we found substantially higher environmental correlations between GAD and MD when GAD was diagnosed non-hierarchically (+0.53) than when diagnosed hierarchically (+0.19) (
Kendler et al, 1992b). The same pattern of results was seen here, but was even more striking. Although the environmental correlation between MD and GAD was quite high (+0.70) when GAD was diagnosed without hierarchy, it did not differ significantly from zero when the DSM-III-R diagnostic hierarchy was imposed. This pattern of results suggests that most of the environmental correlation between MD and GAD may arise from environmental factors that precipitate mixed episodes meeting criteria for both disorders: The environmental risk factors for episodes that are ‘pure’ GAD may bear at most a modest resemblance to those risk factors that precipitate ‘pure’ depressive episodes.
Comorbidity between major depression and other anxiety disorders
Previous analyses in this sample of the causes of comorbidity between MD and phobias (
Kendler et al, 1993) indicate that a high genetic correlation with MD is not characteristic of all anxiety disorders. A recently completed multivariate genetic analysis of lifetime history of MD, GAD, phobias, panic disorder, alcoholism, and bulimia in this female twin sample indicates that the genetic relationship between MD and panic disorder is also relatively modest (
Kendler et al, 1995). This pattern and causes of comorbidity between MD and the anxiety disorders are probably heterogeneous. Our results suggest that, of the anxiety disorders examined in women, only GAD has a close genetic relationship to MD.
Depression, anxiety and stressful life events
Our results suggest that some features of the environment are both aetiologically important for MD and for GAD, and relatively disorder-specific in their impact. Stressful life events (SLEs) are good candidates for such environmental factors. Previous studies have suggested that there is partial differentiation of the kinds of life events that predispose to depression versus anxiety states (
Barrett, 1979;
Finlay-Jones and Brown, 1981;
Cooke and Hole, 1983;
Torgersen, 1985;
Monroe, 1990;
Breslau et al, 1991). In an attempt to replicate and extend these findings, we have begun to explore the relationship between life events and onset of MD and GAD in this twin sample. We have found substantial differences in the pattern of individual SLEs that precede the onset of MD versus GAD. These results tend to confirm the ‘latent’ structural equation mode of environmental risk factors used in this report. When the events are actually measured, as predicted by the latent variable model, the environmental factors that predispose to MD and GAD appear to differ significantly.
Limitations
These results should be interpreted in the context of two potential methodological limitations. Firstly, these analyses had limited statistical power because of the modest one-year prevalence rates for MD and GAD, especially when the latter was diagnosed hierarchically (
Martin et al, 1978;
Neale et al, 1994). Although the total sample size is quite large, most of the information came from affected individuals, which were relatively rare in this sample. Our analyses, therefore, had limited power to detect and estimate parameters accurately. Modest amounts of common environmental variance for MD or GAD, or a modest environmental correlation between the two disorders, could easily have gone undetected in our sample. Furthermore, the confidence intervals around the estimate of genetic correlation between MD and GAD of unity are probably relatively large. Although the necessary power calculations have not been performed, it is unlikely with this sample that we could significantly distinguish a genetic correlation of +0.8 or even +0.7 from unity.
Secondly, our diagnostic approach to GAD was somewhat unconventional. Because of its rarity, we were unable to test GAD using the six-month minimum duration required by DSM-III-R. However, we have previously shown that a multiple threshold analysis suggested that one-month and six-month GAD could be seen as disorders on a single continuum of liability (
Kendler et al, 1992c). Overall, the diagnosis of GAD used in this report was much closer to the DSM-III than the DSM-III-R concept of this disorder. Which diagnostic approach is more valid remains uncertain. The change from a one-month minimum duration of illness in DSM-III to six months in DSM-III-R was made without strong empirical support, and one prior study (
Breslau and Davis, 1985) suggested that this change may have reduced rather than increased the validity of the category. We previously found stronger evidence for heritability for GAD when the minimum duration of illness was one month compared with six months (
Kendler et al, 1992c). Since heritability is an important potential validator of psychiatric diagnoses (
Robins and Guze, 1970), these results also suggest that the DSM-III-R revision may not have improved the validity of the diagnostic category of GAD.