Along with an increasingly sophisticated scientific approach to obstructive sleep apnea has come controversy over its true definition. By convention, the apnea–hypopnea index has been used to characterize obstructive sleep apnea. This index measures the frequency of reductions in airflow associated with upper-airway collapse or narrowing that occurs with the state change from wakefulness to sleep. The term
sleep-disordered breathing encompasses these phenomena. However, a rigid diagnostic standard has not been applied to the apnea–hypopnea index. As a result, its definition differs among sleep laboratories and in the medical literature. A consensus statement was developed over 5 years ago in an attempt to standardize these criteria in human research (
1), and although many sleep laboratories have adopted these recommendations, diagnostic variability remains. According to this statement, an
apnea involves upper-airway collapse and is defined as nearly complete cessation of airflow associated with oxygen desaturation or an arousal from sleep.
Hypopneas, which are associated with partial collapse of the upper airway, should be considered as existing on a pathologic continuum with apneas and may be clinically more important because they may make up the majority of disordered breathing events in a given night (
2).
The apnea–hypopnea index measures the frequency of disordered breathing events but does not quantify other processes that may be operative in the pathophysiology of obstructive sleep apnea, such as the degree of oxygen desaturation. Moreover, the total number of arousals, some of which may occur in the absence of frank breathing abnormalities, may be a superior marker of sleep fragmentation than is the apnea–hypopnea index and may better explain daytime sleepiness (
3). Nevertheless, no other metric has proven to be superior to the apnea–hypopnea index in assessing the overall effect of obstructive sleep apnea, even though it may prove to be inappropriate for characterizing obstructive sleep apnea in specific subsets of patients. For example, it may be that the frequency of arousal (perhaps independent of oxygen desaturation) has the greatest effect on daytime function. Or, perhaps the severity or duration of hypoxemic episodes will be found to have the greatest effect in terms of cardiovascular sequelae.
The obstructive sleep apnea syndrome is defined as sleep-disordered breathing associated with daytime symptoms, most often excessive sleepiness. As accumulating data implicate obstructive sleep apnea in the pathogenesis of systemic disease, particularly in the pathophysiology of cardiovascular disease, the distinction between sleep-disordered breathing and the obstructive sleep apnea syndrome has become increasingly blurred.
Epidemiology
Inconsistencies in definitions of disease and sampling biases contribute to the wide range of prevalence of obstructive sleep apnea reported in the literature. The best evidence of the pervasiveness of obstructive sleep apnea derives from pooled data from 4 large prevalence studies that used similar in-laboratory monitoring, diagnostic criteria, and sampling methods. On the basis of these data, it is estimated that 1 of 5 white adults with an average body mass index of 25 to 28 kg/m
2 has an apnea–hypopnea index of 5 or greater (at least “mild disease”) and 1 of 15 has an apnea–hypopnea index of 15 or greater (at least “moderate disease”) (
4–
8). Up to 5% of adults in western countries probably have the obstructive sleep apnea syndrome (
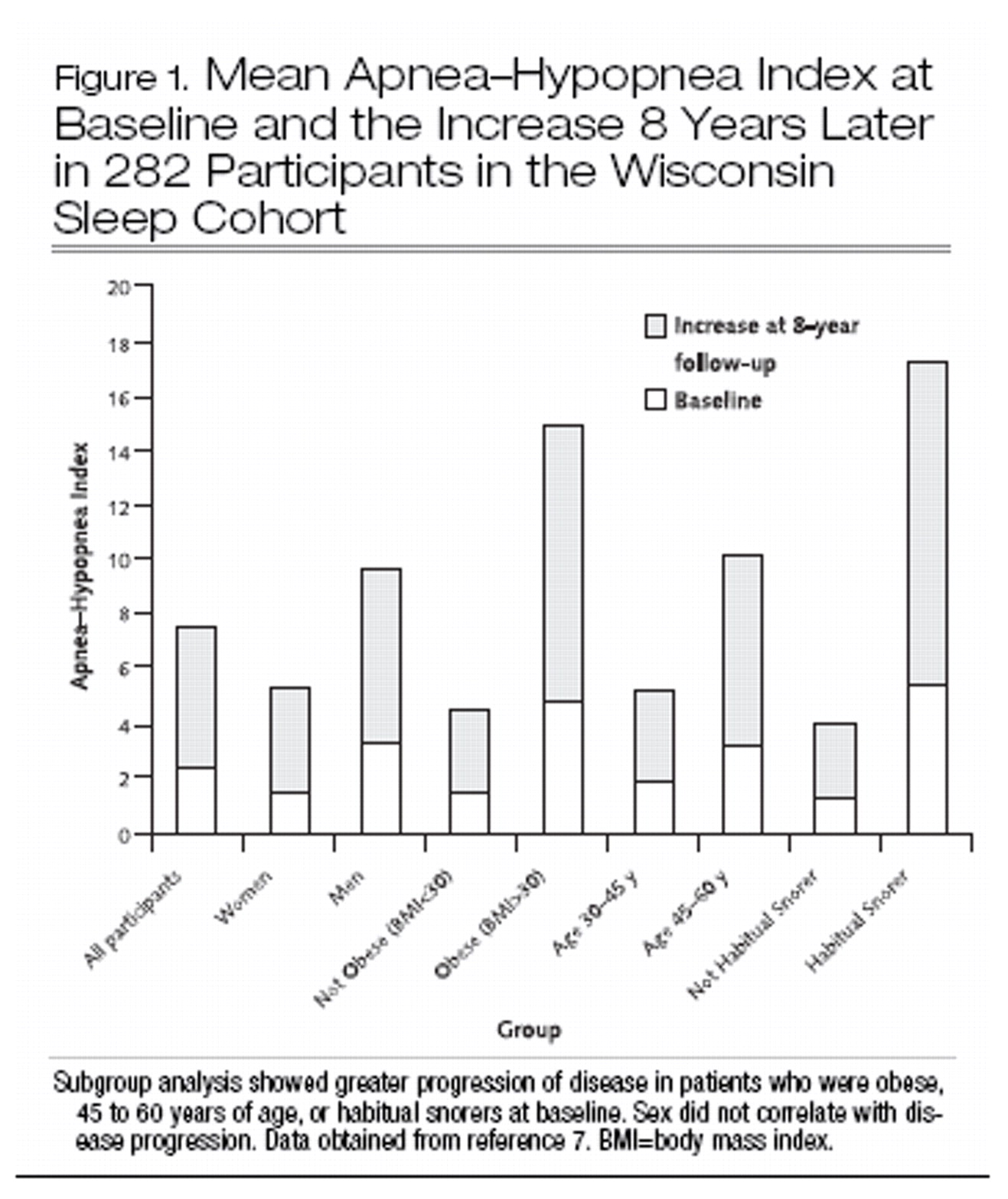
7). Data on the natural history of sleep-disordered breathing from the Wisconsin Sleep Cohort suggest that factors important in progression of disease include baseline obesity, older age, and the presence of snoring (
7) (Figure 1).
Longitudinal data from the Wisconsin Sleep Cohort show that among persons with mild obstructive sleep apnea (apnea–hypopnea index, 5 to 15 ) at baseline, a 10% increase in body weight leads to a 6-fold risk for developing moderate or severe obstructive sleep apnea and a 1% change in body weight predicts a concordant 3% change in the apnea–hypopnea index (
9). The mechanism underlying the risk imparted by obesity is thought to be related, at least in part, to airway narrowing as a result of excess regional soft tissue. The association between neck circumference and the apnea–hypopnea index supports this theory (
10).
Men have a higher risk for obstructive sleep apnea than do women. The reason for this is not entirely clear, but hormonal influences may offer a partial explanation. Postmenopausal women are at higher risk for obstructive sleep apnea than are premenopausal women (
7), an effect that hormone replacement therapy may ameliorate (
11).
The association between age and obstructive sleep apnea is complex. Several studies have shown a higher prevalence of obstructive sleep apnea in elderly persons compared with middle-aged persons, although daytime symptoms may be less common with advancing age (
12). The Sleep Heart Health Study demonstrated that the influence of male sex and body mass index on obstructive sleep apnea tends to wane with age. For unclear reasons, the overall prevalence of obstructive sleep apnea plateaus after 65 years of age (
13).
Data are limited on the occurrence of sleep apnea in nonwhite populations. The prevalence among African-American persons, after adjustment for body mass index, seems to be at least equal to and may exceed that among white persons (
14). The prevalence among men in urban India and men and women in Korea is similar to that observed in western countries (
15,
16).
Clinical presentation
The cardinal symptom of obstructive sleep apnea is excessive daytime sleepiness. It can be difficult to quantify because patients may use varied adjectives to describe sleepiness, and they may confuse sleepiness with fatigue. The challenge for the clinician is to identify sleepiness that warrants further evaluation. Subjective tools, such as the Epworth Sleepiness Scale, are quick and simple to complete but do not correlate well with the severity of the apnea–hypopnea index. It may be the mere presence of sleepiness rather than its quantification that is critical because obstructive sleep apnea correlates with motor vehicle accidents, the risk for which is related to the severity of the apnea–hypopnea index in some (
17), but not all (
18), studies.
Excessive daytime sleepiness appears to result from sleep fragmentation related to recurrent central nervous system arousals in response to disordered breathing events. However, the relationship among these events may be more complex. Experimental disruption of sleep, as manifested by transient blood pressure or heart rate elevations without visible electroencephalographic alterations that traditionally indicate arousal (so-called autonomic arousals), produces evidence of sleepiness on subsequent objective testing (
19). Moreover, although the severity of the apnea–hypopnea index appears to correlate with the degree of subjective sleepiness (
20), many people with an apnea–hypopnea index greater than 5 do not report daytime symptoms. Perception and reporting of daytime sleepiness seem to vary greatly among individuals. When a validated sleep questionnaire was used, a subgroup analysis of the Sleep Heart Health Study showed that 35% of persons with severe obstructive sleep apnea (apnea–hypopnea index ≥ 30) reported sleepiness, as did 21% of those without clinical obstructive sleep apnea (apnea–hypopnea index < 5) (
20).
Obstructive sleep apnea is strongly associated with snoring. Notwithstanding the limitations of quantifying this subjective finding, data from the Sleep Heart Health Study suggest that snoring is associated with daytime sleepiness independently of the severity of the apnea–hypopnea index (
21).
Diagnosis
Several prediction rules have been devised to aid the clinician in determining the pretest probability of obstructive sleep apnea. Such information may allow more efficient use of bed space in sleep laboratories. Validated tools include morphometric examination of the head and neck, which has become more simple and time efficient (
22). Patient questionnaires may perform more powerfully when objective data (particularly anthropometric variables and presence or absence of hypertension) are used along with subjective reporting (
23).
Attended, in-laboratory polysomnography is considered the gold standard in the diagnosis of obstructive sleep apnea. Interlaboratory variation in hardware and assessment methods, however, underscores the need for standardization of criteria to allow comparisons with other emerging diagnostic strategies, such as unattended home monitoring.
Nocturnal pulse oximetry is widely used to screen for obstructive sleep apnea, and some data suggest that it may reduce the need for diagnostic polysomnography in certain circumstances (
24). However, at present, the problems of cost-effectiveness and constraints on bed space in sleep laboratories remain. If the index of suspicion is high, negative findings on pulse oximetry will require confirmatory polysomnography, and positive findings on oximetry studies will require in-laboratory titration of continuous positive airway pressure (CPAP).
Cardiorespiratory physiology of sleep and obstructive sleep apnea
Neural circulatory control during normal sleep
Normal sleep is broadly characterized as rapid eye movement (REM) sleep, which occurs cyclically and more frequently during the second half of the night, and non-REM sleep, which is subclassified into stages 1 through 4 and constitutes the bulk of sleep time. As non-REM sleep progresses, heart rate, blood pressure, and sympathetic nerve activity (as measured by direct intra-neural recordings) decrease (
25). Conversely, REM sleep, which is characterized by increased electrical activity in the brain and sympathetic nerve outflow that can exceed that encountered during relaxed wakefulness, is associated with intermittent and abrupt changes in blood pressure and heart rate (
25). Thus, during normal sleep, stage-dependent modulation of cardiac and vascular physiologic processes occurs. In obstructive sleep apnea, this homeostatic control is severely disrupted.
Airway control in normal sleep and in obstructive sleep apnea
Obstructive sleep apnea is almost exclusively a disease of humans. An exception is the brachycephalic English bulldog, which has been used as a model of upper-airway obstruction during sleep (
26). Observations in this animal model may be applicable to patients with such craniofacial abnormalities as macroglossia, retrognathia, and acromegaly, which are known to predispose to sleep-disordered breathing. The teleologic basis for species selectivity has been theorized to be acquisition of speech, which is necessarily associated with a more compliant upper airway, as demonstrated in the lack of rigid support of the hyoid bone that is specific to humans (
27). That identifiable craniofacial abnormalities account for a minority of patients with obstructive sleep apnea suggests that other pathophysiologic mechanisms are also active.
The inherent collapsibility imparted by the structure of the upper airway is demonstrated by the observation that sleep onset in healthy persons is associated with increased airway resistance (
28) and radiographic evidence of upper-airway narrowing (
29). During normal sleep, various protective mechanisms maintain partial patency of the upper airway. Tonic and phasic activity of more than 20 skeletal muscles that underlie the pharyngeal mucosa play a role in airway dilation and wall stiffening (
30). This activity is further blunted during muscle atonia associated with REM sleep. Chemoreceptor responses to blood oxygen (
31) and carbon dioxide (
32) tensions, as well as local reflex mechanisms, such as the negative airway pressure associated with forceful inspiration, also modulate muscle activity in the upper airway (
33,
34).
Imaging (
35) and endoscopic (
36) studies have shown that during wakefulness and sleep, patients with obstructive sleep apnea have a smaller-caliber upper-airway lumen compared with normal controls. Volumetric magnetic resonance imaging suggests that most of the responsible soft tissue, which may not be entirely explained by fat deposition, originates from the tongue and lateral pharyngeal walls (
37). It appears that this tissue and the narrowing that results render the upper airway of persons with sleep apnea vulnerable to collapse. As a possible compensatory mechanism for this anatomically compromised airway, patients with obstructive sleep apnea have increased electromyographic activity of the pharyngeal dilator muscles during wakefulness (
38,
39).
Pathophysiologic mechanisms during sleep in obstructive sleep apnea
Chemoreflex sensitivity and response to hypercapnia, hypoxia, and apnea
Chemoreflexes mediate the ventilatory response to hypercapnia and hypoxemia. Peripheral chemoreceptors, the most important of which are located in the carotid bodies of the internal carotid arteries, primarily respond to blood oxygen tension (
40), whereas brainstem central chemoreceptors are most sensitive to carbon dioxide and acid–base balance (
41). Even in healthy persons, the chemoreceptor response is blunted during sleep compared with wakefulness, leading to modest changes in blood gas tensions (increase in partial pressure of carbon dioxide of 2 to 6 mm Hg and decreases in oxygen saturation of up to 2%) (
42).
Compared with persons without sleep apnea, patients with obstructive sleep apnea have heightened peripheral chemoreflex sensitivity, resulting in an increased ventilatory response to hypoxemia (
43). This increased response is evident even during normoxia and, by virtue of sympathetic nervous connections with the carotid bodies, contributes to increased sympathetic traffic to skeletal muscle vasculature during daytime wakefulness in persons with obstructive sleep apnea (
44). Further evidence of this phenomenon in otherwise healthy persons with sleep apnea is the inhibition of chemoreflex activity with administration of 100% oxygen, which results in decreases in sympathetic activity, blood pressure, and heart rate (
45). Enhanced chemoreflex sensitivity in obstructive sleep apnea may explain the exaggerated sympathetic response during hypoxemic episodes.
Apnea-induced bradycardia: the diving reflex
In some persons, hypoxemic stimulation of the peripheral chemoreceptors simultaneously increases sympathetic output to muscle and other vascular beds while activation of cardiac vagal activity results in bradycardia. This phenomenon is called the diving reflex, so-named because of its detailed characterization in diving marine mammals. Features of the diving reflex are peripheral vasoconstriction, which preserves blood flow to the brain and heart vessels, and profound bradycardia as a means of limiting cardiac oxygen demand. This protective mechanism allows homeostasis during prolonged periods of apnea and may be activated in some patients with obstructive sleep apnea, manifesting as severe bradyarrhythmias during apneic events.
Baroreflex activation
In healthy persons, there are interactions between the chemoreflex and baroreflex responses. Activation of the baroreflex attenuates ventilatory (
46), sympathetic (
47), and bradycardic (
48) responses to peripheral chemoreflex excitation. Baroreflex dysfunction, such as may occur in hypertension or heart failure, may therefore lessen this buffer effect. This disinhibition of chemoreflex responses may have relevance when cardiovascular disease coexists with obstructive sleep apnea (
48–
50).
Variations in intrathoracic pressure
A physiologic hallmark of obstructive sleep apnea is marked reductions in pleural pressure that are related to respiratory efforts against a narrowed or collapsed airway. The sometimes profoundly negative pleural pressures have important acute mechanical, neural, and circulatory effects (
25). These effects have been simulated experimentally in humans by use of the Müller maneuver, in which the research participant attempts to inspire against a closed glottis. The maneuver (to pleural pressures of −30 cm H
2O) reduces cardiac output and blood pressure, an effect that is more pronounced and prolonged in those with heart failure than in those with normal cardiac function (
51). An experimental canine model of chronic upper airway obstruction during sleep has demonstrated a decrease in left ventricular systolic performance over 1 to 3 months (
52).
Neural circulatory control during obstructive apneas
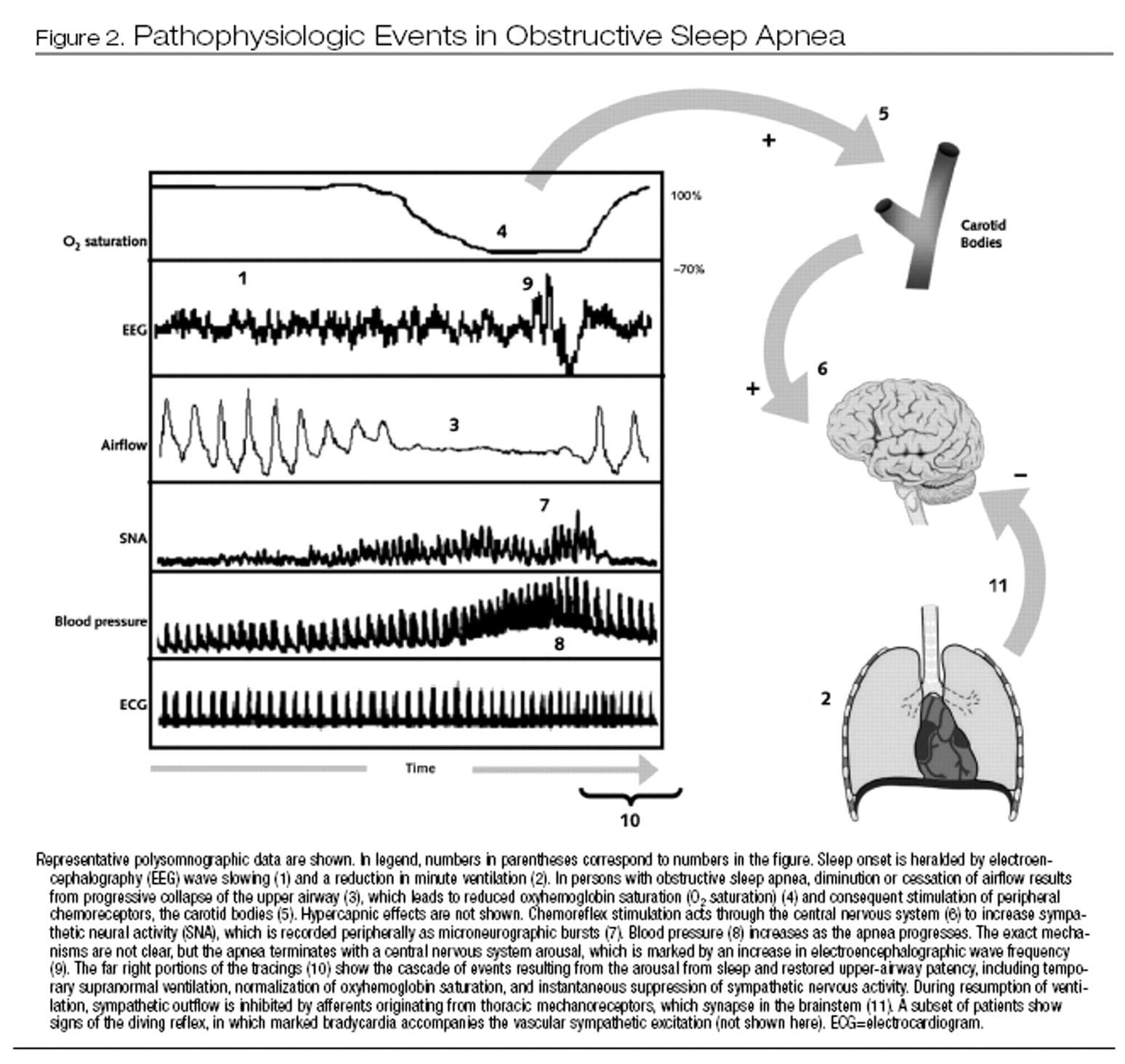
Figure 2 shows some of the pathophysiologic pathways that are activated during an obstructive apneic event. Chemoreflex-mediated vascular sympathetic activation and vasoconstriction intensify as apnea progresses, in association with increases in blood pressure.
Resumption of breathing appears to instantaneously suppress sympathetic activity, for at least 2 reasons. First, lung ventilation activates vagally mediated stretch-sensitive thoracic mechanoreceptors, resulting in sympathetic inhibition. In addition, the surge in blood pressure activates baroreflexes to suppress sympathetic drive. Prevention of repetitive obstructive apneas by use of CPAP attenuates the chemoreflex-mediated sympathetic activation and surges in blood pressure (
53).
Pathophysiologic mechanisms during wakefulness in obstructive sleep apnea
Sympathetic activity and impaired cardiovascular variability
In addition to heightened daytime levels of sympathetic drive, other abnormalities in cardiovascular regulation are observed during resting normoxic daytime wakefulness in patients with obstructive sleep apnea. Compared with controls who have similar blood pressure, patients with obstructive sleep apnea have faster heart rates, blunted heart rate variability, and increased blood pressure variability (
54). The importance of these factors in obstructive sleep apnea is yet to be determined, but large studies have shown them to be markers of increased cardiovascular risk in the general population (
55).
Vascular dysfunction
Impaired endothelial function, which has been demonstrated experimentally as an attenuated vasomotor response to vasoactive stimuli, may be an independent marker of future risk for cardiovascular events (
56). The physiologic stressors associated with apneas cause elaboration of endogenous vasoactive substances, of which the most important may be endothelin, a potent, long-lasting vasoconstrictor. After about 4 hours of disordered breathing events during sleep, endothelin levels and blood pressure increase (
57). Use of CPAP reverses the increases in endothelin levels and blood pressure for several hours. Furthermore, levels of nitric oxide, a powerful vasodilator, may be decreased in obstructive sleep apnea (
58) Overnight use of CPAP has been shown to increase circulating levels of nitric oxide (
59).
Systemic inflammation
Serum levels of the inflammatory markers C-reactive protein (
60) and serum amyloid A (
61) appear to be increased in persons with sleep apnea and may be pathophysiologically related to sleep deprivation or intermittent hypoxemia (
62,
63). Moreover, there is evidence of oxidative stress in obstructive sleep apnea (
64). These inflammatory pathways are thought to play a role in the development and progression of cardiovascular disease (
65) and may be important mediators of disease in obstructive sleep apnea.
Metabolic dysregulation
After adjustment for body weight, patients with obstructive sleep apnea have a higher prevalence of insulin resistance and diabetes mellitus than do healthy persons (
66). The relationship among these conditions may be critical when making associations with cardiovascular disease. The etiologic mechanism of insulin resistance may be linked to sleep deprivation or sympathetic activation in patients with obstructive sleep apnea (
67). Abolition of sleep-disordered breathing by the use of CPAP mitigates glucose intolerance in both the short term (
68) and the long term (
69). This effect wanes with increasing body weight (
68).
Regulation of leptin, the adipocyte-derived protein product of the
ob/ob gene, also appears to be abnormal in patients with obstructive sleep apnea (
70). Leptin is known to act in the central nervous system as an appetite suppressant, and its blood concentrations correlate closely with overall adipocyte mass. Hyperleptinemia in obstructive sleep apnea may reflect target tissue resistance to the weight-reducing effects of leptin (
70).
The complex interaction between obstructive sleep apnea and obesity is underscored by the strong predisposition to weight gain in the year leading up to diagnosis (
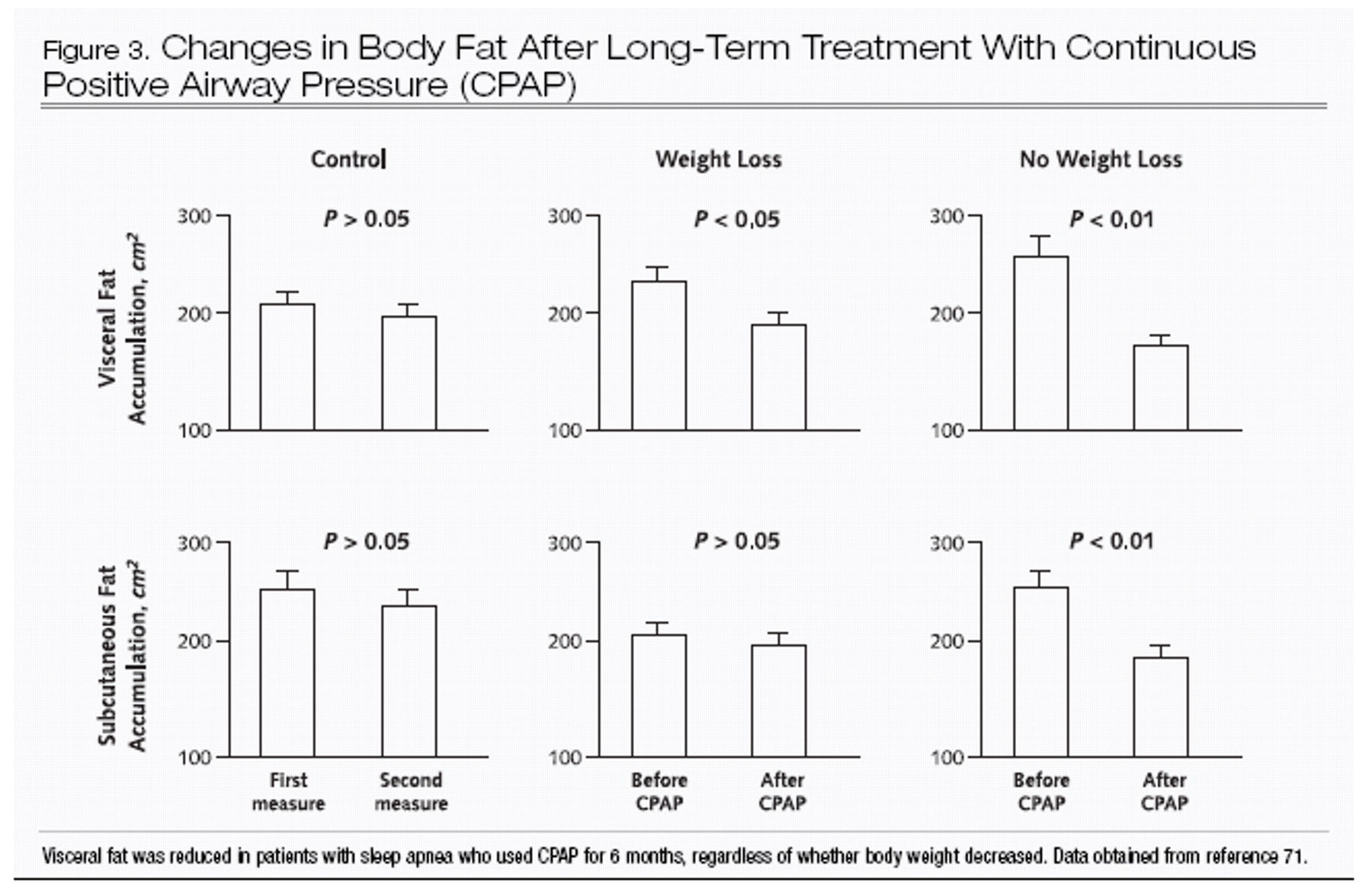
70). Recent data suggest that treatment of obstructive sleep apnea with CPAP will decrease not only leptin levels but also central obesity, independent of any overall change in body weight after treatment (
71) (Figure 3).
Cardiovascular diseases related to obstructive sleep apnea
For nearly 3 decades, research studies have suggested an association between sleep apnea and cardiovascular disease and have implicated obstructive sleep apnea in the causality of systemic disease. However, in early reports, this association was often confounded by other comorbid conditions, most notably obesity. Case–control and cross-sectional studies were limited by the inability to prove that obstructive sleep apnea preceded the development of cardiovascular disease. With the recognition of these limitations have come more rigorous methods, including longitudinal cohort studies and interventional clinical trials.
Hypertension
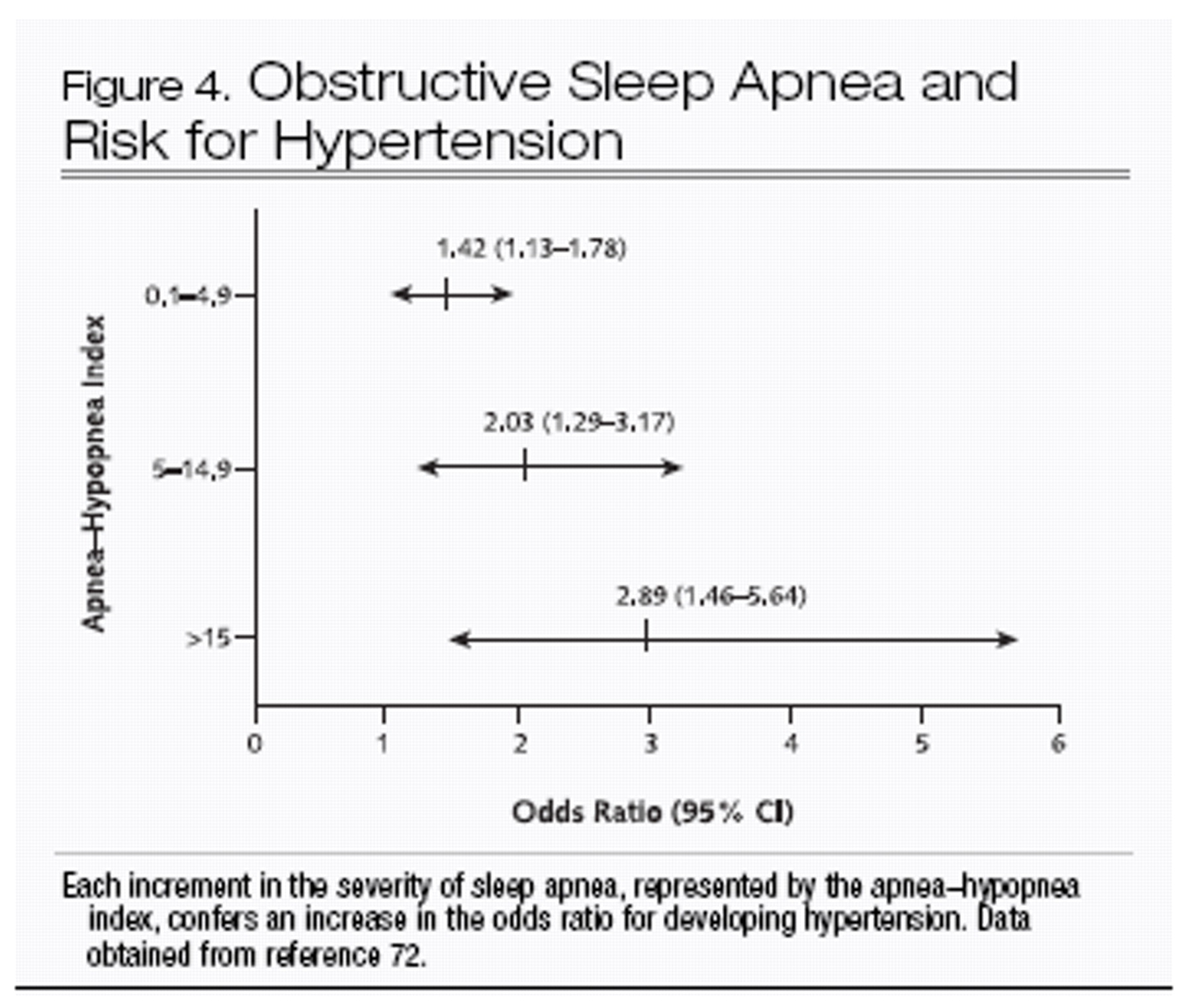
The most compelling evidence that obstructive sleep apnea is causally related to cardiovascular disease comes from the Wisconsin Sleep Cohort Study (
72). In normotensive persons who were followed for 4 years after an initial sleep study, worsening severity of obstructive sleep apnea was independently associated with progressively increasing risk for new hypertension (Figure 4). Even persons with very mild abnormalities in the apnea–hypopnea index (0.1 to 4.9) had 42% greater odds of developing hypertension at follow-up than did those with an apnea–hypopnea index of 0. Thus, the aforementioned physiologic mechanisms that contribute to acute increases in blood pressure during sleep-related breathing events may also affect daytime blood pressure.
The greater question may be whether treatment of obstructive sleep apnea has efficacious antihypertensive effects. Small randomized studies of therapeutic versus sham CPAP in normotension and mild hypertension have shown a small but significant effect in decreasing nighttime and daytime blood pressure (
73,
74).
Heart failure
Available evidence suggests that at least 10% of patients with heart failure have clinically significant obstructive sleep apnea (
75). Some data show that nocturnal upper-airway edema in patients with congestive heart failure may predispose to or worsen obstructive sleep apnea by narrowing the airway lumen (
76). For unclear reasons, patients with coexisting obstructive sleep apnea and heart failure may be less likely to report substantial daytime sleepiness (
77).
Because hypertension is a common cause of heart failure, the association between obstructive sleep apnea and hypertension (
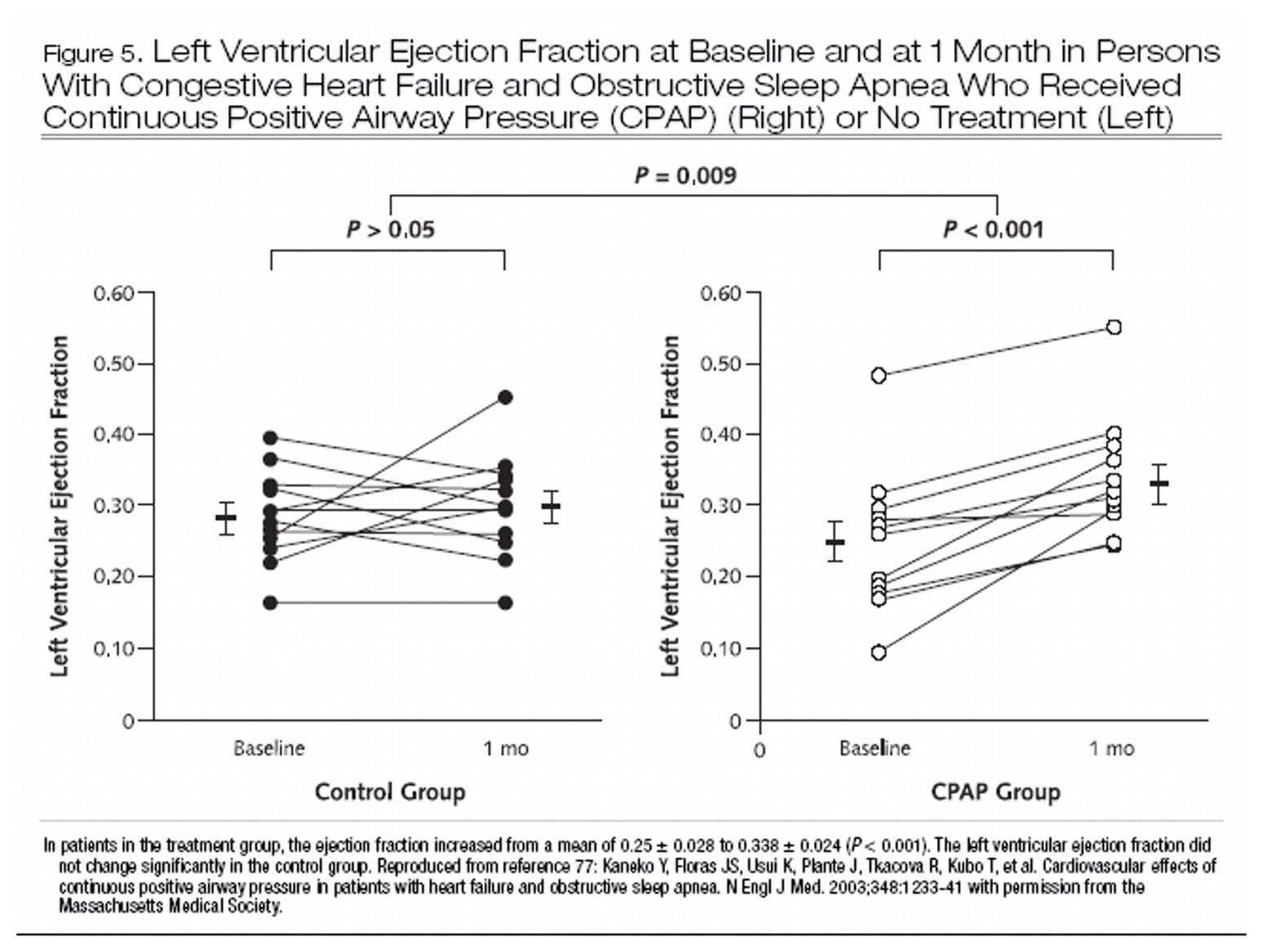
72) may be a powerful indirect risk factor for congestive heart failure in patients with obstructive sleep apnea. Although large-scale randomized trials of treatment of obstructive sleep apnea in patients with heart failure are lacking, smaller studies have suggested improvement in surrogates of outcome, such as left ventricular ejection fraction (Figure 5) and blood pressure (
77), as well as decreases in urinary cate-cholamine levels (
78). Whether the mortality rate is reduced, however, remains unknown.
Cardiac arrhythmias
Use of a validated screening tool suggests a high prevalence of obstructive sleep apnea in patients with atrial fibrillation (
79) and, among patients who underwent cardioversion for atrial fibrillation, the recurrence rate in those with untreated obstructive sleep apnea was nearly double that seen in those treated with CPAP during 1 year of follow-up (
80). Further research is needed to determine the independent effects of obstructive sleep apnea on atrial fibrillation, because both conditions share common associated diseases, including hypertension and heart failure.
Marked bradycardia, asystolic episodes, and ventricular arrhythmias have been documented in patients with obstructive sleep apnea, even in the setting of normal cardiac electrical conduction (
81). The clinical importance of these arrhythmias, and whether they are related to sudden cardiac death, remains to be determined.
Management
Because a decrease in body weight of as little as 10% has been associated with clinically significant improvement in the apnea–hypopnea index (
82), weight loss should be recommended to overweight patients with obstructive sleep apnea. Although the amount of weight loss seems to correlate directly with reduction in sleep-disordered breathing (
7), studies have demonstrated modest improvement in rather than normalization of the apnea–hypopnea index. The long-term effects of increasingly popular methods of weight loss, such as bariatric surgery and carbohydrate-restricted diets, on the severity of obstructive sleep apnea must be assessed in longitudinal studies.
Continuous positive airway pressure remains the most effective therapy for obstructive sleep apnea. It eliminates upper-airway flow limitation in almost any patient and is associated with improvement in daytime symptoms (
83) and objective measures of sleepiness (
84) in patients with mild or severe abnormalities in the apnea–hypopnea index. Use of CPAP mitigates or reverses many of the acute pathophysiologic responses outlined previously that result from sleep-disordered breathing. Currently, however, few data support use of CPAP in the absence of daytime symptoms. Randomized clinical trials suggest that sleepiness may be an important manifestation of systemic disease. For example, use of CPAP to treat sleep-disordered breathing has been shown to have little effect on blood pressure in patients who are not sleepy (
85).
On the basis of its established effectiveness in ameliorating sleep-disordered breathing and reversal of daytime symptoms, a trial of CPAP therapy is warranted in most patients with the obstructive sleep apnea and associated daytime symptoms. Universal adherence to this therapy is hampered by intolerance or refusal in a substantial proportion of patients. Nonadherence is often related to nasal congestion and irritation, which may be ameliorated by in-line heated humidification and local treatments, such as moisturizers or corticosteroids.
The role of surgery in the treatment of obstructive sleep apnea remains ill defined and is controversial. The most appropriate indication is clearly reversible causes of upper-airway obstruction, such as adenotonsillar hypertrophy or mass lesions. Application of surgical therapy to general populations with obstructive sleep apnea is limited by a dearth of randomized, controlled trials to support its routine use. Uvulopalatopharyngoplasty (also known as UPPP), a commonly performed inpatient procedure involving excision of soft palatal tissue, results in an apnea–hypopnea index less than 20 in a minority of patients (
86). The suboptimal performance of uvulopalatopharyngoplasty may be explained in part by the role of anatomic factors other than soft palatal tissue. Imaging studies (
29) and airway pressure measurements (
76) have revealed that the oropharyngeal and velopharyngeal areas, which are dorsal to the soft palate and tongue base, respectively, are the regions most vulnerable to collapse during sleep.
Even in patients in whom sleep-disordered breathing seems to improve in the early postoperative period after uvulopalatopharyngoplasty, these effects may be short-lived. Thus, polysomnography should be repeated within 4 to 6 months.
Mandibular advancement devices, which induce protrusion of the mandible during sleep and thereby reduce retroglossal airway collapse, appear to be effective in the treatment of mild apnea or nonapneic snoring and have been shown to improve daytime symptoms and modestly decrease the apnea–hypopnea index (
87,
88).
Summary
Obstructive sleep apnea remains an important public health problem because of its neurocognitive sequelae. Detailed characterization of physiologic pathways and cardiovascular disease mechanisms suggest an important role of obstructive sleep apnea in systemic disease, particularly of the cardiovascular system. Rigorous studies with a refined diagnostic approach to obstructive sleep apnea are needed to identify the relative importance of apneas, hypopneas, blood gas abnormalities, arousals, and sleep deprivation in the initiation of cardiovascular disease. An added challenge will be disentangling the influence of multiple comorbid conditions that are often present in patients with obstructive sleep apnea.
Small interventional studies suggest that CPAP reverses some of the important pathophysiologic pathways in obstructive sleep apnea, but this finding has not been clinically validated by large-scale randomized, controlled trials. Further clarification of physiologic and pathologic pathways may help to identify novel mechanism-based therapies that interrupt or delay progression of pathophysiologic processes related to obstructive sleep apnea.