History
Recognizable in centuries old writings, obsessive-compulsive disorder (OCD) was often thought to be an affliction requiring religious intervention (
1). Bishop John Moore certainly seems to have been describing obsessive-compulsive patients who
“… [Have] naughty, and sometimes Blasphemous Thoughts [which] start in their Minds, while they are exercised in the Worship of God [despite] all their endeavors to stifle and suppress them … the more they struggle with them, the more they encrease.” [Moore 1692/1982, quoted in Pitman (
1, p. 4)]
Even earlier, Bishop Jeremy Taylor [1660/1982, quoted in Pitman (
1, p. 4)] had referred to William of Oseny who
“read two or three books of Religion and devotion very often … [he] had read over those books 3 hours every day. In a short time he had read over the books three times more…. [He] began to think … that now he was to spend 6 hours every day in reading those books, because he had now read them over six times. He presently considered that … he must be tied to 12 hours every day.”
Later, OCD and attendant tics were viewed as manifestations of eccentricity. The renowned lexicographer Samuel Johnson (1709–1784) was described by a friend: “ … he whirled and twisted about to perform his gesticulations; and as soon as he had finish'd, he would give a sudden spring and make such an extensive stride over the thresholds, as if he were trying for a wager how far he could stride. …” (
2). Esquirol (
3) described a woman with “monomaniacal” obsessions and rituals. Westphal (
4) gave a clear description of the syndrome. Janet (
5) and Freud (
6) also provided phenomenological descriptions of OCD, which still ring true today.
Etiology
Theories of etiology often imbue treatments with merit more apparent than real. Sir Clifford Allbutt's (
7) observations about the etiology of atherosclerosis also apply to OCD:
It is when we come to study the causes of diseases that we are most disconcerted by the breadth and depth of our ignorance; it is here that our path is most cumbered with guesses, presumptions and conjectures, the untimely and sterile fruitage of minds which cannot bear to wait for the facts, and are ready to forget that the use of hypotheses lies not in the display of ingenuity but in the labor of verification.
Early theories of demonic possession gave way gradually to others. Freud (
6) described anal eroticism, orderliness, parsimony, and obstinacy from the time of toilet training, which he believed emerged in later life as obsessions and compulsions reflecting issues of aggression, sexuality, and control from that earlier epoch.
Behaviorists emphasize anxiety acquisition through the pairing of a neutral event with an aversive experience that evokes anxiety or other discomfort. Behaviors (avoidance and observable or mental rituals) that decrease anxiety or other discomfort are repeated because of their beneficial effect but become problems in their own right as they prevent habituation and become excessive (
8).
Abnormalities of serotonin neurotransmission are current popular explanations of OCD and are amenable to many biochemical research paradigms (
9). Anatomic circuits and metabolic abnormalities have been hypothesized or demonstrated (
10), and there is an active pursuit of many lines of research that contribute to an understanding of the abnormalities associated with OCD. It is tempting to speculate that, regardless of the anatomic, metabolic, and biochemical derangements of OCD, the problem may be one of severity and not of type. The main themes of obsessions lie on the dimension of danger, and patients feel compelled to perform rituals that lessen those anxieties, albeit incompletely. There is an obvious evolutionary advantage in being appropriately concerned about danger and in checking, cleaning, grooming, washing, counting, ordering, straightening, and storing enough but not too much (
11,
12).
The undoubted mediation of OCD through gene expression begs the question, which genes? Although evidence is beginning to emerge that specific genes are involved in OCD, it is probable that different combinations of genes will be involved even in phenotypically similar OCD. For example, assessment of serotonin system candidate genes in 54 parent-child trios showed no evidence for association at any of the polymorphisms in any subjects, perhaps because of low power across individual association studies (
13). Even more recently, glimmerings of genetic understanding have emerged: “Using compulsive hoarding as the phenotype, there was suggestive linkage to chromosome 14 at marker D14S588 (Kong and Cox logarithm of the odds ratio [LOD] [KAC
all=2.9]). In families with two or more hoarding relatives, there was significant linkage of OCD to chromosome 14 at marker C14S1937 (KAC
all=3.7), whereas in families with fewer than two hoarding relatives, there was suggestive linkage to chromosome 3 at marker D3S2398 (KAC
all=2.9)” (
14). Notably, this article listed 22 authors from five universities, and there was an accompanying editorial to further describe the meanings, promise, and limitations of our present understanding. Still, the future timing of definitive elucidation of pathological gene patterns remains indeterminate, and the ability to manipulate gene function to therapeutic advantage is still further removed.
The complexity of genetic research is reflected in a “Perspective” in a recent issue of the
New England Journal of Medicine: “For the clinician, the outcome of these (genome-wide association) studies will be similar to those of any new investigative technology. The first results will need to be verified in similar populations by independent groups. Then, the usefulness of the variants for clinical practice will depend on their improving diagnostic prediction or fostering changes in prevention or treatment strategies. There is a compelling need for more, and more efficient, epidemiological studies so that these new approaches can be exploited. To meet this need, scientists and clinicians must collect information, informed consent, and tissue samples in the expectation of future studies that will address as yet unformed questions” (
15).
It is helpful for patients and clinicians to have a conceptual etiological model on which to hang their hats. Although oversimplified, concern for safety with avoidance of excessive harm, risk, and danger is probably normally distributed in the population. Under the broad part of a bell-shaped curve, this characteristic conveys a survival advantage for the individual and the genes he or she carries. At either tail of the curve, with excessive or negligible concern for safety, gene transmission is reduced. Those with OCD and excessive concern about safety (e.g., contamination obsessions about sex) are less likely to mate and reproduce, whereas those without fear may not live long enough to reproduce.
Phenomenology
As its name implies, OCD is characterized by obsessions and compulsions.
Obsessions are unwanted intrusive ideas, images, or impulses that patients often experience as senseless, thereby demonstrating that they have preservation of insight and are not delusional. Patients often feel besieged by their incessant obsessions, confirming the appropriateness of the derivation from the Latin
obsidere, which means “to besiege.” The most common obsessions involve themes of harm, risk, or danger, which include contamination, doubt, aggressivity, disorder, and loss of things of importance.
Compulsions are urges that patients experience to lessen their anxiety, discomfort, dysphoria, or feelings of disgust that usually emanate from obsessions. Compulsions typically lead to performance of repetitive, purposeful, intentional behaviors called
rituals. Rituals help individuals achieve transient reduction in discomfort from obsessions, but at a cost of having to be done to excess. Washing and cleaning usually relieve concerns about contamination; checking reduces doubts; avoiding objects of aggression or the implements with which one might be aggressive allays anxiety about aggression; straightening and ordering decrease discomfort from disorder; and hoarding counteracts fears of losing things.
Avoidance of situations that would evoke obsessions and consequent rituals and various forms of
checking summarize many subtypes of obsessions and rituals and should be sought in the mental status examination as signals of OCD (
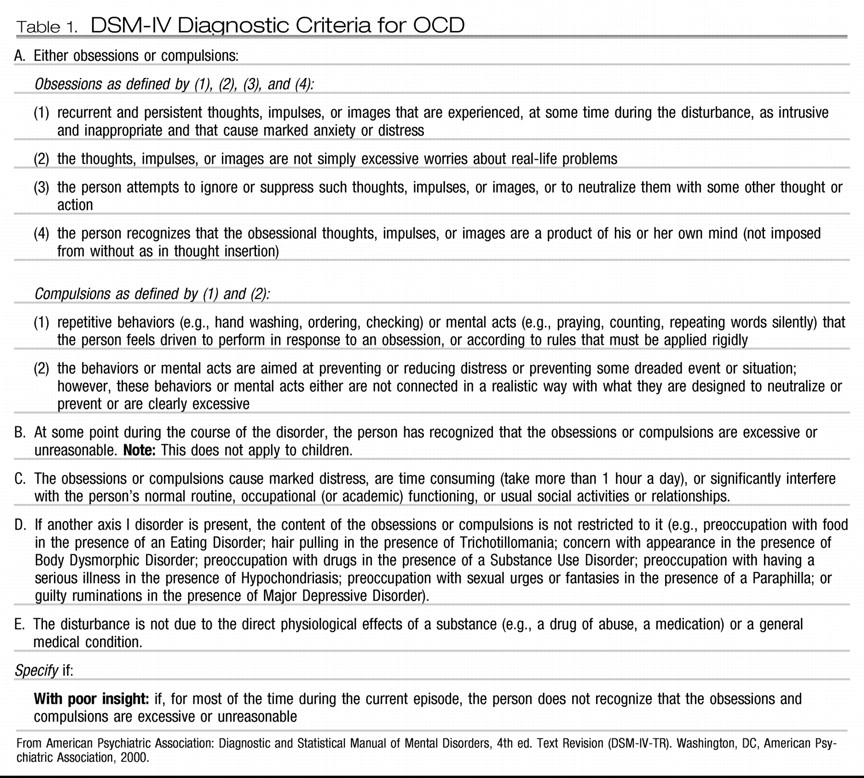
16). The DSM-IV-TR (APA) diagnostic criteria for OCD are presented in
Table 1.
Although ritualistic behaviors are often observable, they may take the form of mental behaviors. Thus, a parent obsessed with horrific images of slaughtering her family members may repeat, sotto voce, “God would never let me harm my family, God would never let me harm my family.” Rituals must be repeated because patients feel uncertain that they have done enough to prevent harm, risk, or danger. These major themes account for about 80% of obsessions and compulsions, but the protean nature of obsessions and the rituals that relieve them are legendary, and most patients have several obsessions and compulsions. Fewer than 5% of patients have only obsessions or compulsions, and these singular claims are suspect and often overlook mental rituals that are confused with obsessions because both occur as thoughts. The distinction is easily made: obsessions are thoughts that intrude and are anxiogenic; mental rituals are mental behaviors performed by the person and are anxiolytic.
Many disorders share phenomenological similarities with OCD. Patients with hypochondriasis are obsessed with the idea that they have a dread disease and compulsively seek repeated examinations from physicians to diagnose the malady. When the physician reassures them that there is no evidence of disease, they are relieved, but only briefly, because reassurance, as other rituals, provides no lasting benefit. Somatization disorder, body dysmorphic disorder, eating disorders, nail biting, and some impulse control disorders (e.g., trichotillomania, kleptomania, and pathological gambling) (
17) are sometimes conceptualized as part of an obsessive-compulsive spectrum. Some spectrum patients respond to the same treatments effective in patients with OCD.
Most patients with OCD hide their obsessions and rituals because they fear stigmatization should they be discovered. Jenike (
18) has called OCD a hidden epidemic. Few patients disclose obsessions and compulsions directly to physicians, but many complain about comorbid conditions (
19). Two thirds of patients with OCD have a major depressive episode during their lifetimes. Other anxiety disorders and Gilles de la Tourette syndrome are much more common in patients with OCD than in the general population (
Table 2) (
20). Personality disorders, particularly cluster C (anxious-fearful) disorders, occur in about 60% of patients with OCD (
21).
Epidemiology
OCD is quite common, with 1-month and lifetime prevalences of 1.3% and 2.5%, respectively (
22,
23). Early onset is common, with mean ages in males and females of 18 and 21 years, respectively. Prepubertal onset is frequent, and some grade-school children are incapacitated to the point that they cannot attend school (
11,
12). The most recent National Comorbidity Survey Replication (
24) found a consonant 12-month prevalence of 1.0% with 51% of patients with “serious” severity defined primarily as 30 days or more out of their major role in the year.
After onset, OCD usually runs a chronic course of waxing and waning severity; few patients have spontaneous remissions, and about 10% experience a devastating downhill course until their lives become dominated by the disorder. On average, patients who seek treatment have waited a decade after symptoms emerged before doing so. OCD is a familial disorder; slightly more than 20% of an index patient's family members also have OCD, and others have stigmata of the disorder but do not meet full diagnostic criteria (
19). OCD occurs in all cultures and races, although some cultures continue to believe that OCD is a religious affliction. Other cultures believe that individuals with OCD are merely willful, and still others view OCD as an illness or disorder.
Pathophysiology
Many biological aspects of the pathophysiology of OCD are being studied intensively. Different imaging techniques and biochemical assays and probes generally support the following findings:
1.
Orbitofrontal cortex, anterior cingulate cortex, and caudate nuclei of patients with OCD exhibit increased metabolism compared with control subjects (
25,
26).
2.
Effective short-term treatment with a selective serotonin reuptake inhibitor (SSRI) [fluoxetine (Prozac)] or behavior therapy (exposure and response prevention) (
10) reduces hypermetabolism in the right caudate nucleus, whereas effective long-term treatment with a potent serotonin reuptake inhibitor (SRI) [clomipramine (Anafranil)] reduces hypermetabolism in the orbitofrontal cortex (
27).
3.
Serotonin is significantly involved in OCD because
A.
Potent SRIs are effective treatments for OCD.
B.
Metachlorophenylpiperazine (mCPP), a serotonin agonist, exacerbates obsessions and rituals in some patients with OCD.
C.
This mCPP effect can be blocked by effective treatment with clomipramine and fluoxetine.
4.
Potent noradrenergic reuptake inhibitors such as desipramine (Norpramin) are ineffective treatments for OCD.
Animal models for OCD suggest possible pathophysiology in humans and potentially useful treatments. Schedule-induced polydipsia in rats has been shown to respond to clomipramine, fluoxetine, fluvoxamine (Luvox), and all potent SRIs, and it does not respond to desipramine, haloperidol (Haldol) (a dopamine antagonist), or diazepam (Valium) (a benzodiazepine) (
28). Psychogenic polydipsia is a sometimes lethal disorder most often seen in patients with schizophrenia. Hyponatremia, hypo-osmolality, and cerebral edema are caused by compulsive fluid consumption, with seizures, coma, and death occurring in some patients. One patient with a severe case responded well to treatment with fluoxetine (
29). Canine acral lick, an excessive grooming behavior in large dogs, also responds to potent SRIs (
30).
Knowledge is accumulating about the anatomy, metabolic abnormalities, and neurochemistry of OCD, but the pathophysiology of the disorder is not definitively understood (
31).
Assessment
Several assessments of the nature and severity of OCD are available, although most practitioners have neither time, training, nor inclination to use them, trusting instead their more global impressions. The merit of measurement is real and can be realized by turning the task over to the patient who is the subject expert on symptomatology. Sharing this burden adds further value as patient and clinician have more consensus on status, and this factor has been shown to increase treatment efficacy (
33).
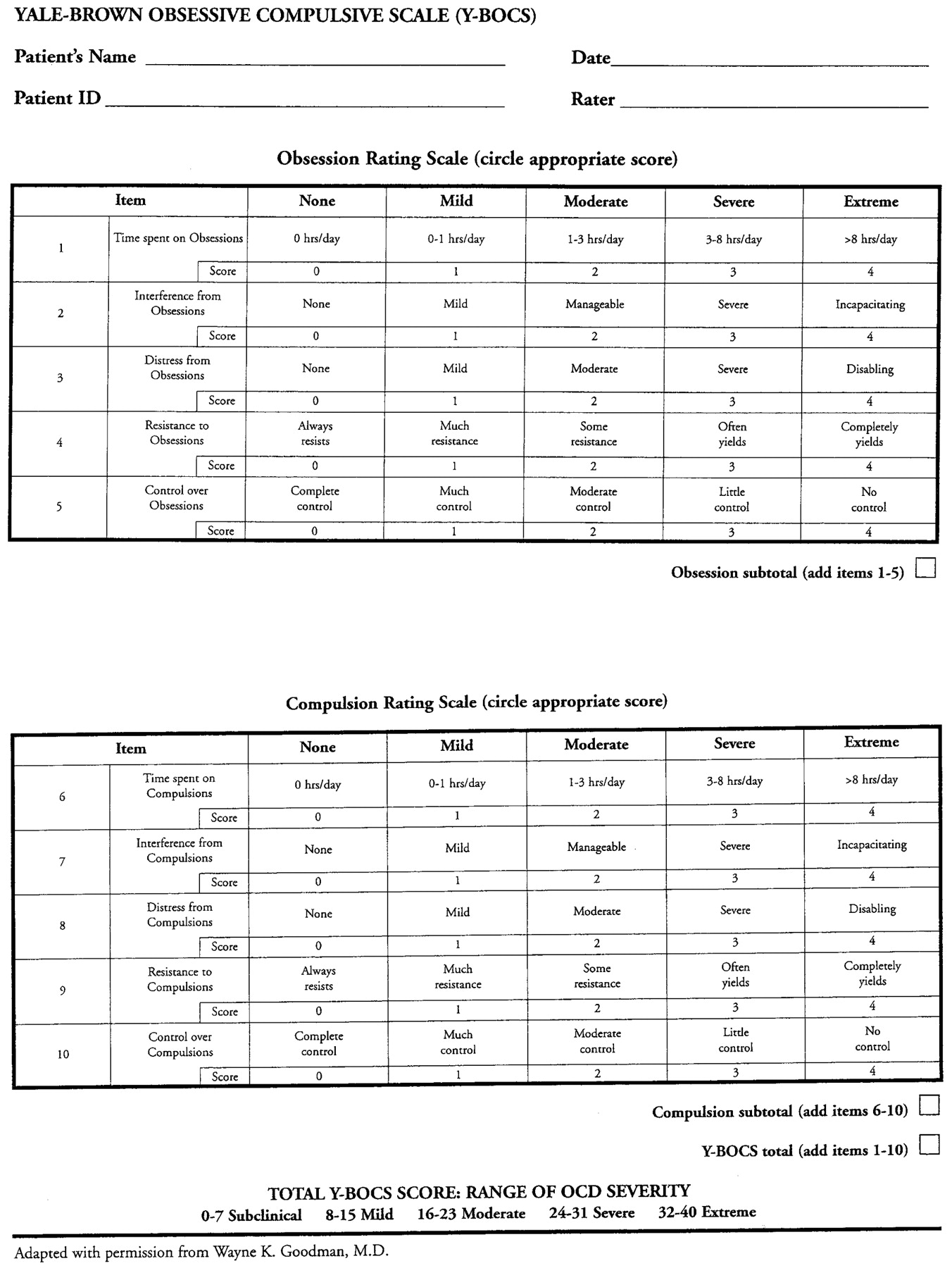
The gold standard Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) (see
Appendix A) was used in all randomized controlled trials that led to Food and Drug Administration (FDA) medication approvals for OCD. There is also a children's version (CY-BOCS). Ten items, five for obsessions and five for compulsions/rituals can be scored by clinicians or equally well by almost all adult patients using either a paper and pencil or computer interview versions (
34). Scores ≤10 indicate subclinical illness, 11–16 mild illness, 17–24 moderate illness, 25–32 marked illness, and 33–40 severe illness.
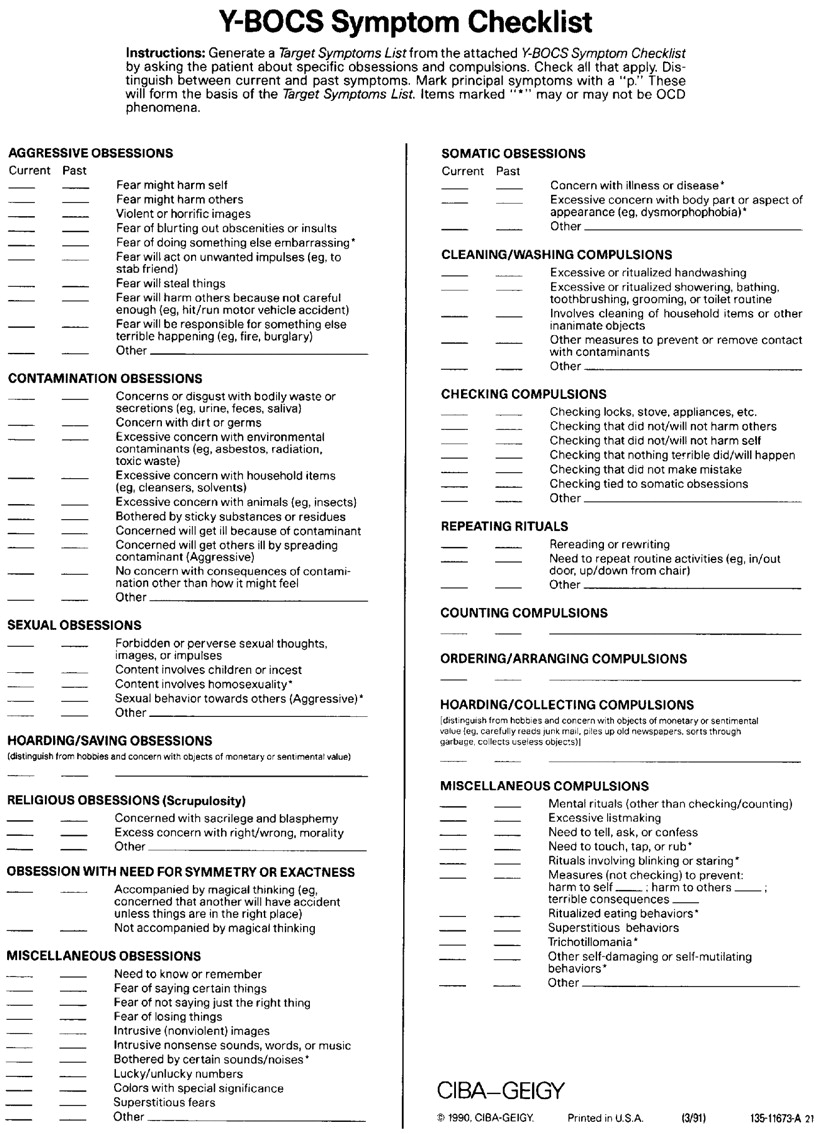
The Y-BOCS Symptom Checklist (see
Appendix B) identifies more than 60 obsessions and rituals and is very helpful in defining the extent of past and present pathology. Asking patients to indicate severity for each present obsession and ritual on a 0 (none) to 10 (most severe imaginable) scale permits refined follow-up of change as treatment proceeds. These two self-report Y-BOCS assessments provide accurate information about the patient's OCD and effectiveness of treatment. Other assessments may be used but are not as widely recognized or understood, and we prefer the Y-BOCS.
Treatment
Until 1966, OCD was properly considered a refractory disorder for which only neurosurgery was inconsistently effective. “Despite Freud's acclaimed success in curing the ‘Rat Man' (
6), analytic approaches have not been studied systematically or shown to be helpful, and the limitations of this approach are often acknowledged by analysts: ‘It is apparently true, for instance, that there is no known authenticated case of an obsessive handwasher being cured by psychoanalytic treatment'” (
35,
36). Trials of a broad range of psychopharmacological agents were equally ineffective.
In 1966, clomipramine was released in Switzerland, and behavior therapy was tried in OCD. Both were dramatically beneficial against the backdrop of previous treatment failures (
37,
38). As with many defining psychiatric therapies emerging in that era (e.g., chlorpromazine for schizophrenia and lithium for bipolar disorder), little, if any, greater efficacy has emerged since then beyond the marginal advantage of multiple medications with similar mechanisms but different structures, each helping fractionally more patients, and hard won nuanced knowledge about pharmacokinetic and pharmacodynamic effects of medication combinations. SSRIs do have a better side effect and safety profile than clomipramine and are preferred as first line drug treatment for that reason.
Behavior therapy
Following Meyer's lead, clinicians have conducted numerous randomized clinical trials that confirm the principal value of exposure and response (now called
ritual) prevention as the behavioral treatment of choice for OCD. Greater short-term (
39–
42) and lasting benefits (
43) of behavior therapy compared with benefits with medications is well documented. The value of this behavior therapy for children, adolescents, and adults is recognized in all treatment guidelines for OCD (
44–
49). Two landmark studies summarize what is known without oversimplifying complexity that requires individualization of treatments.
In adults, Foa et al. (
50) compared clomipramine with behavior therapy, their combination, and pill placebo as 12-week treatments for OCD. A figure in their article [which is reprinted in this issue beginning on page
368] confirms the chronicity of OCD treated with placebo and shows that clomipramine was significantly more efficacious than placebo and that behavior therapy was significantly more efficacious than clomipramine, whereas the combination was not better than behavior therapy alone.
In children and adolescents, the Pediatric OCD Treatment Study (POTS) (
51) compared sertraline with cognitive behavior therapy (CBT), their combination, and placebo pill, also for 12 weeks. Results were quite similar to those found in the Foa et al. (
50) study in adults.
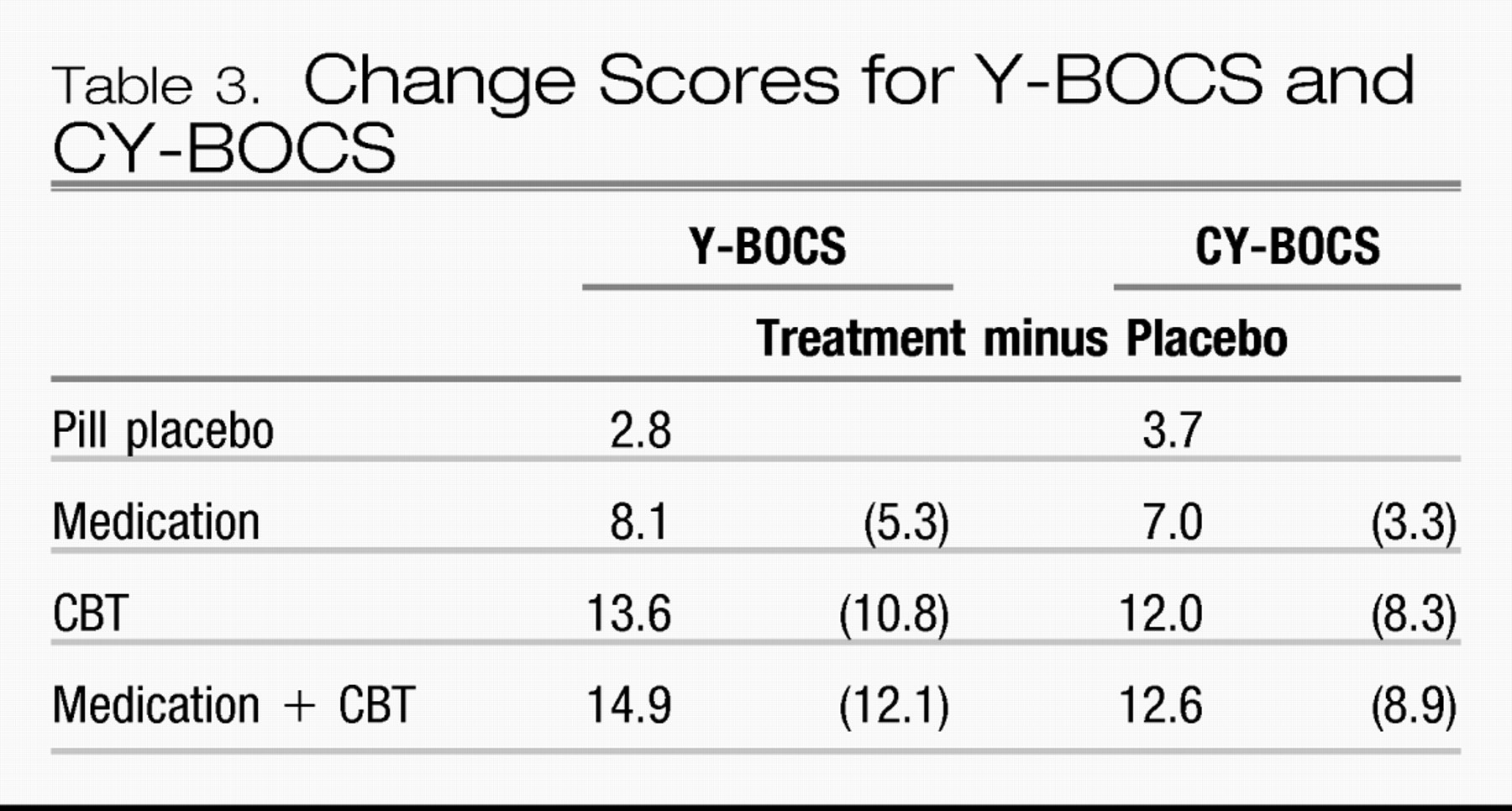
Table 3 shows change scores for the Y-BOCS and its child version CY-BOCS.
Forty hours of face-to-face behavior therapy was used in the adult study and 14 hours in the child study. In both studies, treatment-placebo numeric improvement on the Y-BOCS was at least twice as large for CBT as for the FDA-approved SRI medication against which CBT was compared. Combined treatment was little better than CBT alone. The combination did result in lower medication doses in both studies (163 mg/day in combination with CBT versus 196 mg/day for clomipramine monotherapy in adults and 133 mg/day versus 170 mg/day for sertraline in children). The POTS study reminds us of Disraeli's dictum that “There are three kinds of lies: lies, damn lies, and statistics,” as statistical analysis showed the combination to be significantly better than CBT alone based on a numeric difference of 0.6 (out of more than 8 points on the Y-BOCS), whereas the larger numeric difference of 1.3 in the adult study did not! Site variability in results of CBT was significant in the POTS study. The CBT effect size at the Penn site (1.6) was more than three times larger than that at the Duke site (0.51), whereas medication differences were smaller (0.53 and 0.8, respectively). This suggests that therapy standardization is more difficult with CBT than with medication.
Despite the well-documented and marked advantage of CBT over medication for OCD, the likelihood of receiving CBT treatment ranged from only 5% (
52) to 38% in the Brown (University) Longitudinal Obsessive Compulsive Study (
53), although only 24% in the Brown study had at least 13 treatments within 1 year. Further, some patients have only relaxation, which is used as a placebo in randomized, controlled trials (RCTs) of other elements of CBT. The authors of the seminal adult and child studies shared similar laments:
“Expertise in exposure and ritual prevention is uncommon among clinicians in the community, perhaps because typical psychotherapists, even with cognitive behavior therapy training, do not treat sufficient numbers of OCD patients to acquire the experience necessary for effective intervention for patients with difficult-to-treat OCD. One solution is to develop regional specialty clinics similar to centers for heart disease and cancer (
50).”
“Unfortunately, despite ready availability of the CBT protocol, only a small minority of children and adolescents with OCD receive state-of-the-art treatment(s) for reasons that may include features of the intervention itself as well as variables pertaining to the practitioner, client, model of service delivery, organization, and service system…. In this context, it is imperative that the focus of research turn to identifying and testing dissemination strategies for CBT as well as to procedures for managing partial response to medication monotherapy using CBT augmentation (
51).”
Training more therapists to use these efficient, effective, straightforward techniques would be highly valuable but achieving such providential improvement after three decades of failed attempts would be akin to the leopard changing its spots.
One approach to making scarce CBT skills more widely available is therapy provided largely by telephone. After a single face-to-face assessment and introduction to CBT in trials in three different settings, weekly phone contacts over 8, 10, or 12 weeks lasted 15, 30, and 45 minutes, respectively, to design and encourage exposure and ritual prevention homework (
54–
56). Immediate posttreatment Y-BOCS reductions of 12.0, 9.2, and 10.9 were found, respectively, the last in a RCT comparing telephone and face-to-face CBT in which Y-BOCS score reduction was 10.3 when it was administered by the same therapists. Follow-ups of 1, 3, and 6 months, respectively, found maintenance of improvement. Satisfaction with telephone CBT was as high as with face-to-face CBT.
An alternate approach that provides effective behavior therapy without requiring clinicians to change how they practice is also possible. BT STEPS, a computer-assisted exposure and ritual prevention program teaches patients how to systematically self-manage their behavior therapy. In a RCT (
57), 64% of patients did at least one exposure and ritual prevention homework session and decreased their Y-BOCS score by 7.8 points. Those randomly assigned to behavior therapists for 12 hours of face-to-face individual sessions decreased their Y-BOCS score by 8.1 points. There was a strong dose-response relationship with BT STEPS CBT. For those who did between 2 and 20 homework sessions the Y-BOCS score decreased 10.3 and with more than 20 sessions the score decreased 14.3 points. These improvements occurred without therapist coaching or support. Addition of these elements by scheduled telephone calls in another study increased the percentage of patients using BT STEPS, who did at least two exposure and ritual prevention sessions, from 57% to 95% and more than doubled Y-BOCS improvement (
58). Therapist telephone calls averaged 13 minutes each, with a total mean time of 76 minutes per patient.
Although unstudied, the simplest approach to providing CBT where none is available is to give literate patients a self-help book to guide their efforts. A few determined patients have done well through this medium alone although a modicum of coaching and support from a clinician would probably speed and increase improvement. Clinicians also find these brief books useful sources of education (
59–
64).
Cognitive therapy
Cognitive therapists seek to change thoughts, feelings, and behaviors. They hypothesize that faulty cognitions permit and then maintain unpleasant affects and dysfunctional behaviors. Correcting faulty cognitions should lead to more agreeable affects and more functional behaviors. However, it is striking that in OCD, patients with insight have called their cognitions crazy thousands of times, family members have argued against their obsessional fears, and clinicians have agreed that their worries are unfounded, all without benefit.
Reviews (
65,
66) have documented efficacy for cognitive procedures that address faulty risk assessment and exaggerated sense of responsibility. Whether cognitive therapy might achieve its benefits directly through cognitive means or, indirectly, by invoking exposure and ritual prevention is an important question (
67).
Medications
“If many treatments are used for a disease, all are insufficient” as stated by Osler (
68).
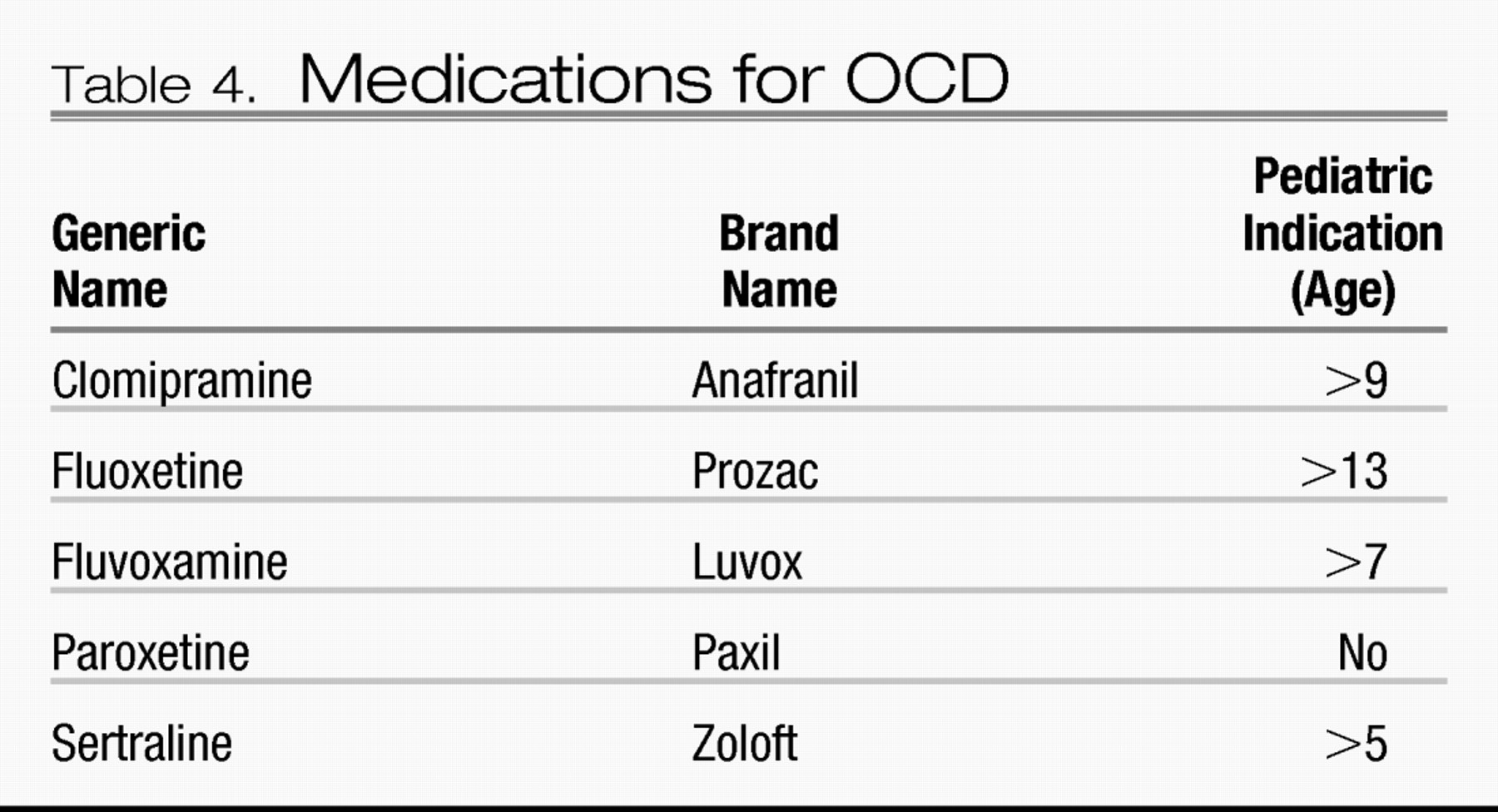
Several medications have received FDA indications for treatment of OCD in adult and pediatric populations (
Table 4). Although other SSRIs and the serotonin-norepinephrine reuptake inhibitor venlafaxine do not have FDA-approved indications for OCD, citalopram, escitalopram, and venlafaxine have demonstrated efficacy in RCTs and may reasonably be tried off-label after due consideration has been given to SRIs with FDA approvals. For example, escitalopram was as efficacious as paroxetine at 24 weeks in achieving ≥25% reductions in Y-BOCS scores (
69), and both were more efficacious than placebo. In a 6-month, placebo-controlled relapse prevention study (
70), relapse was 2.74 times greater with placebo than with escitalopram.
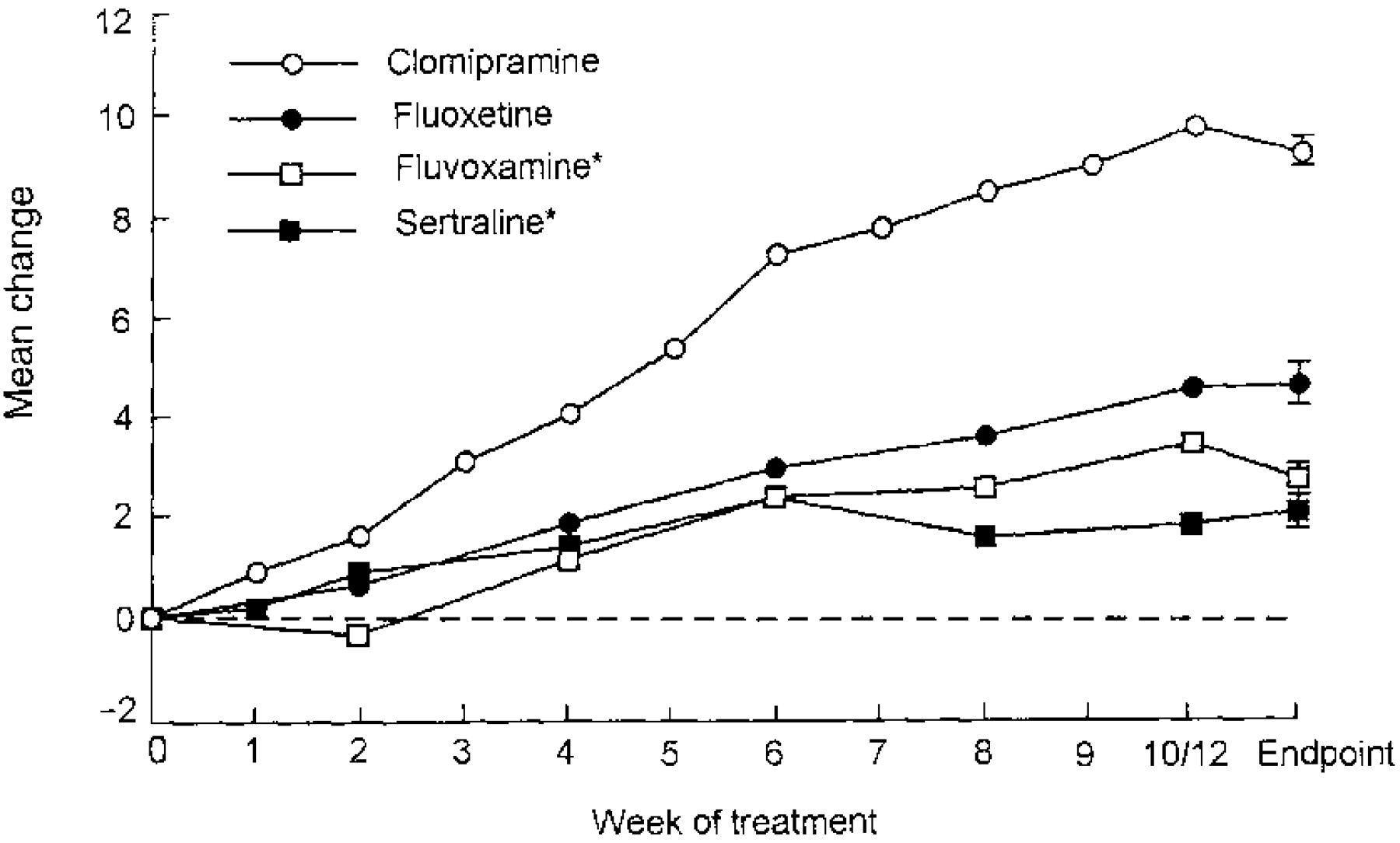
Clomipramine has demonstrated greater efficacy than SSRIs for adults in meta-analyses in which they have been compared (
71–
74) (
Figure 1). Clomipramine also showed significantly greater efficacy than fluoxetine (p<0.03), fluvoxamine (p=0.001), paroxetine (p=0.003), and sertraline (p<0.001) in a meta-analysis of 1,044 children and adolescents with OCD (
75). But as a tricyclic antidepressant, clomipramine also conveys a heavier side effect burden. Thus, SSRIs are the first-line medication treatment for OCD, with clomipramine usually being reserved for trials in patients who show insufficient improvement with a first or second SSRI. With SSRIs, it is impossible to predict responses, with the rare exception of identical twins, so any SSRI may be used first. We give consideration to patient preference, having provided information about likely limiting side effects including weight gain, discontinuation difficulty, and possible fetal risk during pregnancy and, importantly, cost. When SRIs are discontinued, rapid relapse is the rule rather than the exception (
76,
77), an unfortunate occurrence in sharp contrast to CBT, for which gains to 6 years have been documented (
43).
Dosing
Many psychiatrists believe, with little supportive evidence, that OCD treatment requires large or even heroic dosing to achieve best results. This belief appears to arise from several sources and is understandable, well intentioned, and misguided. Before the availability of potent SRIs, there was no effective pharmacotherapy for OCD and that experience led some to the superficially logical but incorrect conclusion that whatever medication proved effective in OCD would need to be given in large doses. SSRIs have a large therapeutic index so high doses were more tolerable than with clomipramine for which the therapeutic index is narrow, limited both by typical tricyclic side effects and risk of seizure and cardiac arrhythmias. Despite the markedly better response to potent SRI antidepressants than the poor response to previously tried non-SRI monotherapies, remission virtually never occurred in adult patients, so the dose was increased in hopes of further benefit. Finally, with the higher doses a few patients did benefit, possibly because of particular pharmacokinetic or pharmacodynamic factors. These exceptions were thought to prove the rule that higher doses were better; but the rule they prove is that humans, in general, do a poor job of estimating complex risks, and human clinicians are no exception to this rule. Thus, there was no dose-response relationship in FDA OCD registration trials for fluoxetine or sertraline and no difference between 40 and 60 mg/day for paroxetine, with 40 mg/day being the lowest effective dose.
Although a significant dose-response relationship for efficacy does not exist with SRIs in OCD, the adverse effects dose-response relationship is clear. Children with OCD precipitated by pediatric autoimmune neuropsychiatric disorders associated with streptococcus may be especially vulnerable to behavioral activation with high SRI doses (
78).
A method that avoids the risk of excessive dosing while acknowledging the fact that a few individuals need larger doses than the norm is:
•.
to begin with a small, but usually effective, dose (e.g., fluoxetine 20 mg/day), watch for a response and if the response occurs within 4 weeks, and
•.
wait for the beneficial response to plateau, based on Y-BOCS scores, many weeks later.
•.
At that point, dose should be increased (e.g., to fluoxetine 40 mg/day) to determine whether the patient benefits from a higher dose, again based on the Y-BOCS score. If further improvement results, the process is repeated until no further improvement is found or side effects limit any further dose increase.
•.
If no improvement occurs with the dose increase, the dose is systematically reduced to the previous effective dose, thereby minimizing risk of side effects and cost.
•.
This approach optimizes the dose, ensuring maximum improvement while minimizing side effects. Considering the chronicity of OCD, this deliberate approach to optimal dosing is appropriate.
Beyond basic psychopharmacology for OCD
Given the proven efficacy of SRIs in OCD, proserotonergic augmentation theories were logically advanced, and treatments derived from them were tested. Open trials suggested promise with the recommendation for placebo-controlled RCTs, which, unfortunately, showed no significant benefit from buspirone (
79–
81) or lithium (
82,
83). Within the active treatment arms, some patients had some benefit so these augmentations may be worth trying in patients with partial, but insufficient, responses to SRIs alone.
Open-label combinations of clomipramine with fluvoxamine (
84) and citalopram (
85) produced greater improvement than monotherapies. Pharmacokinetic mechanisms were suggested to explain the fluvoxamine combination, and pharmacodynamic mechanisms were suggested for citalopram.
Best established are augmentations of SRIs with antipsychotics for treatment-resistant OCD. Following the placebo-controlled RCT with haloperidol augmentation of fluvoxamine of McDougle et al. (
86), which showed clear benefits in patients with OCD and comorbid tics, other RCTs of antipsychotic augmentation have been conducted. By March 2006, 10 trials had been reported with haloperidol (N=1), olanzapine (N=2), risperidone (N=3), and quetiapine (N=4). These were subjected to a meta-analysis (
87). The weighted combined response ratio with 157 patients receiving drug and 148 receiving placebo was 3.31 (95% confidence interval 1.40–7.84). Antipsychotic doses were modest and, within this low dose range, there may have been a dose-response relationship. In individuals with schizophrenia with and without comorbid OCD, atypical antipsychotic drugs have sometimes been associated with exacerbation or onset of OCD symptoms (
88–
92).
An open trial suggestion of benefit from SRI augmentation with oral morphine given weekly in treatment-resistant OCD (
93) was confirmed in a placebo- and lorazepam-controlled RCT (
94). The weekly administration interval suggested a mechanism different from that involved in pain relief.
Other somatic therapies
Electroconvulsive therapy was often tried in severe OCD before effective treatments became available, and sometimes since, and patients occasionally benefitted (
95,
96). Responding patients probably had primary depression with secondary OCD symptoms, or they may have had exacerbation of primary OCD when a secondary depression, which occurs commonly, was superimposed. There is little indication and no convincing evidence that primary OCD responds to electroconvulsive therapy.
An open trial suggested that 20 minutes of right lateral prefrontal transcranial magnetic stimulation (TMS) at 80% of the motor threshold dose produced a reduction in obsessive-compulsive symptoms lasting 8 hours. Left lateral prefrontal TMS was ineffective and midoccipital stimulation increased obsessive-compulsive symptoms (
97). Another open-label series also supported efficacy of TMS (
98). However, three sham-controlled repetitive TMS studies (two right and one left prefrontal stimulation locations) found no difference between repetitive TMS and sham treatment arms (
99–
101). These results emphasize again the frequent difference between open and randomized, controlled trials of treatments and the high value for the profession and patients of publishing negative trial results.
An open trial of vagus nerve stimulation (
102) in seven patients with treatment-resistant OCD was conducted. Acutely and at 6 months, three of seven patients (43%) had ≥25% reductions in Y-BOCS scores.
Neurosurgery, properly reserved for the most severe and incapacitating OCD, was sometimes beneficial before SRIs and CBT were available and has continued to prove helpful for a few patients when thorough trials of these favored treatments were truly ineffective (
103). Anterior cingulotomy, subcaudate tractotomy, and their combination as limbic leucotomy as well as anterior capsulotomy were all used and created lesions in pathways between brain structures shown to be functioning abnormally in OCD. With modern neurosurgical techniques including stereotactic control, prospective studies have confirmed benefits (
104,
105) and postoperative hemorrhage, infection, and seizures are uncommon, mortality is rare, and personality change, when it occurs, is usually favorable (
106). Preoperative positron emission tomography scans of the right posterior cingulate cortex may identify patients more likely to have a good response to anterior cingulotomy (
107).
Deep brain stimulation via removable electrical leads placed bilaterally in the anterior limb of the internal capsule (one site of effective ablative procedures) has proven helpful for a small but growing number of patients with carefully documented intractable OCD (
108–
110) The largest series, combined from three pilot studies in Antwerp/Leuven, Providence, RI/Cleveland, OH, and Gainesville, FL, has followed 26 patients (
111). Overall the mean Y-BOCS score decreased >35% and more than 50% of patients achieved this excellent outcome as well as a substantial increase in Global Assessment of Functioning scores from a baseline of 25–41 to a score >50. Twelve patients have been followed for >3 years with lasting improvements unless stimulation was interrupted. Few adverse events, none serious, were reported across the full population. There were no group-level neuropsychological declines after a mean of 10 months of stimulation in the 10 Providence, RI/Cleveland, OH, patients. Surprisingly, visuospatial skill, visual learning, and story memory improved.
All of these other somatic therapies remain experimental and require subspecialty knowledge, skills, and specific written informed consent for their application.
Conclusions
We have emphasized results from RCTs, where available, to provide evidence-based guidance for treatment of OCD. These emphases are expressed mainly in mean scores or average results. But none of our patients is average—each is an individual, and these data on mean effects represent only a starting point from which to plan individual treatment.
Getting long in the tooth ourselves, we look back across decades of preoccupation with pathophysiology and continued fascination with mechanisms of action of medications at the expense of translating what is known empirically about treatment into helpful practice. We struggle to adhere to Carlyle's admonition: “Our main business is not to see what lies dimly at a distance, but to do what lies clearly at hand” (
112). However, none of this criticism diminishes the importance of research that will ultimately improve our understanding of OCD and its treatments.
What are the best treatments we can offer patients with OCD based on what we know now? All are best served if we understand and accept proven knowledge, including physicians' inability to apply what we know is best treatment. Particularly annoying is our failure to accept, adopt, and apply CBT as the treatment of choice for OCD.
Table 3 shows that treatment-minus-placebo Y-BOCS reductions with CBT are more than double those with clomipramine and sertraline in the definitive adult and pediatric OCD trials (10.8 versus 5.3 and 8.3 versus 3.3, respectively). In addition, relapse after medication discontinuation is the rule, but relapse after CBT is uncommon.
That our reach always exceeds our grasp is not unique to psychiatrists. In 1976, internal medicine residents complied with only 22% of 390 obvious and important practice protocols (
113). Their compliance improved to 55% after introduction of an innovation, but returned to baseline when it was removed. Twenty-five years later in the same institution, influenza immunizations were provided to only 1% of inpatients aged 65 and older until the innovation was used and raised immunization rates to 55% (
114). The innovation was computer reminders to the doctors regarding their own practice protocols. The amount they still fell short of their practice goals was attributed to “the nonperfectability of man.” We care about influenza immunizations because poor practice produces poor outcomes. Immunization of elderly individuals reduces hospitalizations for cardiac and cerebrovascular disease by 19%, for pneumonia or influenza by 30%, and for all causes deaths by 49% (
115).
For those wanting to offer the best treatment to patients with OCD who want it, CBT with exposure and ritual prevention is the answer. The essence is exposure to triggers of anxiety followed by foregoing rituals that reduce anxiety—a treatment that must be self-administered so the clinician's role is to guide and coach the patient. Once established, CBT becomes a way of life, changing inappropriate risk aversion to therapeutic risk taking, where the risks are not really risky, and providing large and lasting improvements.
Successive trials of SRIs, dosed properly for sufficient duration and then continued indefinitely, is also effective treatment for OCD. The magnitude of SRI benefits (effect size) is about half that for CBT, and benefits usually disappear shortly after medication is discontinued. Augmentation with atypical antipsychotic drugs appears helpful, and other combinations with less evidence supporting their efficacy may also help some patients. The side effect burden of medications must be managed as well as ongoing cost.
Patients who have access to CBT and medication sometimes do better than either alone and with CBT, have the possibility of discontinuing medication without substantial worsening of OCD.
For patients with intractable and incapacitating OCD, neurosurgical lesions have sometimes been helpful. Deep brain stimulation in the same areas has demonstrated improvement to at least 3 years without irreversible lesions.
And finally, we advocate measuring treatment effects systematically and sharing assessment results with the patient. The patient is the subject expert on his or her symptoms and functioning, so repeated patient-completed Y-BOCS scores are ideal. This simple step yokes doctor and patient into a strong team to lessen the heavy burden of OCD.