Delirium, defined as an acute and sudden change in attention and overall cognitive function, is a substantial medical problem for older persons—and one that may be preventable. Patients ages 65 years and older account for almost half (49%) of all days of hospital care, and although delirium is the most frequent complication affecting this population, it often goes unrecognized. In fact, delirium affects over 2.5 million patients ages 65 and older during hospitalization annually (
Inouye et al. 1999;
U.S. Department of Health and Human Services 2004). Delirium is a costly condition, leading to increased costs per hospital stay of at least $2,500 per patient, which translates to $6.9 billion (values in
U.S. dollars in 2004) of annual excess Medicare hospital expenditures directly related to delirium and its complications. Patients with delirium have a worse prognosis than patients without delirium and are at an increased risk of developing long-term cognitive and functional decline (
Inouye 2006;
Jackson et al. 2004), which in turn leads to additional posthospitalization treatment costs, such as for institutionalization, rehabilitation services, and home health care (
Inouye 2006). Total health care costs related to delirium are estimated at $38 billion to $152 billion annually (
Leslie et al. 2008).
DEFINITION AND EPIDEMIOLOGY
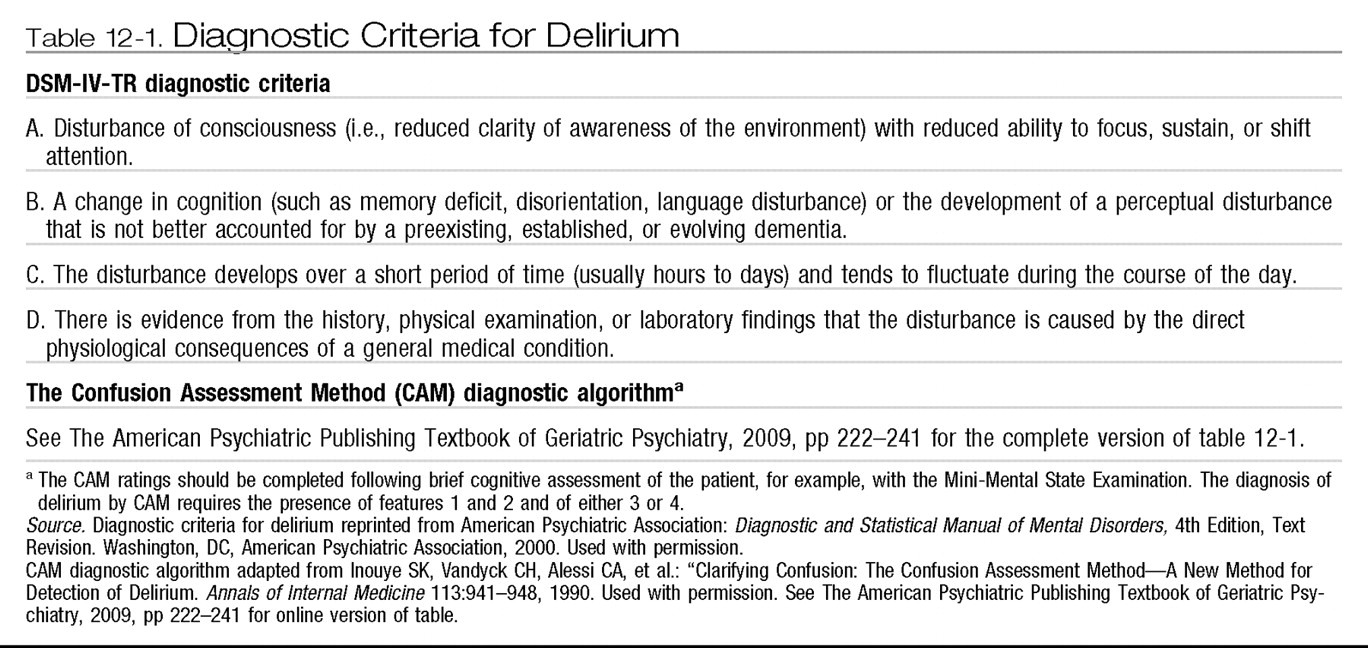
The diagnostic criteria for delirium that appear in DSM-IV-TR (
American Psychiatric Association 2000) are generally accepted as the current diagnostic standard (see
Table 12-1). Expert consensus was used to develop the DSM-IV-TR criteria, and sensitivity and specificity estimates of the criteria have not been reported. The Confusion Assessment Method (CAM) (
Inouye et al. 1990) provides a simple diagnostic algorithm that has become widely used as a practical means for identification of delirium (see
Table 12-1). The CAM diagnosis of delirium is based on an assessment of the clinical features of acute onset and fluctuating course, inattention, disorganized thinking, and altered level of consciousness. The CAM algorithm has a sensitivity of 94%–100%, specificity of 90%–95%, positive predictive accuracy of 91%–94%, and negative predictive accuracy of 90%–100% compared with the ratings of geropsychiatrists, as well as high interrater reliability (
Inouye et al. 1990).
Delirium is often the only sign of an acute and serious medical condition affecting a patient, and it most commonly occurs in frail older persons with an underlying disease process. Occurrence estimates suggest that delirium affects 14%–56% of hospitalized elderly patients (
Cole 2004). Delirium is a symptom in up to 30% of older patients presenting to the emergency department (
Agostini and Inouye 2003;
Inouye 2006). Delirium following surgery is common in patients ages 65 and older, occurring in 15%–53% postoperatively (
Balasundaram and Holmes 2007;
Inouye 2006;
Olin et al. 2005). Not surprisingly, incidence rates increase to 70%–80% of older patients in intensive care (
Pisani et al. 2003) and to over 50% of those in nursing home or post-acute care settings (
Kiely et al. 2004,
2006). Following hospitalization, the estimated 1-year mortality rate for patients with delirium is 35%–40% (
Moran and Dorevitch 2001).
CLINICAL FEATURES AND COURSE OF DELIRIUM
Sudden and acute onset, alteration in attention, and fluctuating course are the central features of delirium. Therefore, it is important to establish a patient's level of baseline cognitive functioning and the course of cognitive change when evaluating for the presence of delirium. A detailed and in-depth background interview with a proxy informant, such as a family member, caregiver, or medical professional who knows the patient, proves invaluable when documenting change in a patient's mental status. It is important to differentiate between 1) cognitive changes that increase and decrease in severity over a period of days, which is indicative of delirium, and 2) changes that are more chronic and progressive over a period of months to years, which is indicative of dementia. To fulfill the criteria for delirium, the change in cognitive status must occur in the context of a medical illness, a metabolic disorder, drug toxicity, or drug withdrawal.
The cognitive evaluation for delirium should encompass the following domains: global cognitive changes, impairment in attention, disorganized thought process, and altered level of consciousness. Global cognitive changes associated with delirium can be assessed through simple cognitive testing and close clinical observation during test administration and the patient's completion of tasks. It is important not to underestimate the waxing and waning periods of delirium, because periods of lucidity and reversal of symptoms can often be deceiving. Impairment in attention, a hallmark feature of delirium, is clinically manifested through the patient's difficulty focusing on the task at hand, maintaining or following a conversation, and/or shifting attention, often leading to perseveration on a previous topic or task. Disorganized thought is present when the patient's speech is incoherent or jumbled and when the patient lacks a clear or logical presentation of ideas; this problem can be similar to the “word salad” phenomenon seen in schizophrenia and other formal thought disorders. Alteration in consciousness is highly variable and can range from an agitated or aggressive state to one of lethargy or stupor. Other clinical features commonly associated with delirium that are not included in the diagnostic criteria are psychomotor agitation, paranoid delusions, sleepwake cycle disruption, emotional lability, and perceptual disturbances or hallucinations.
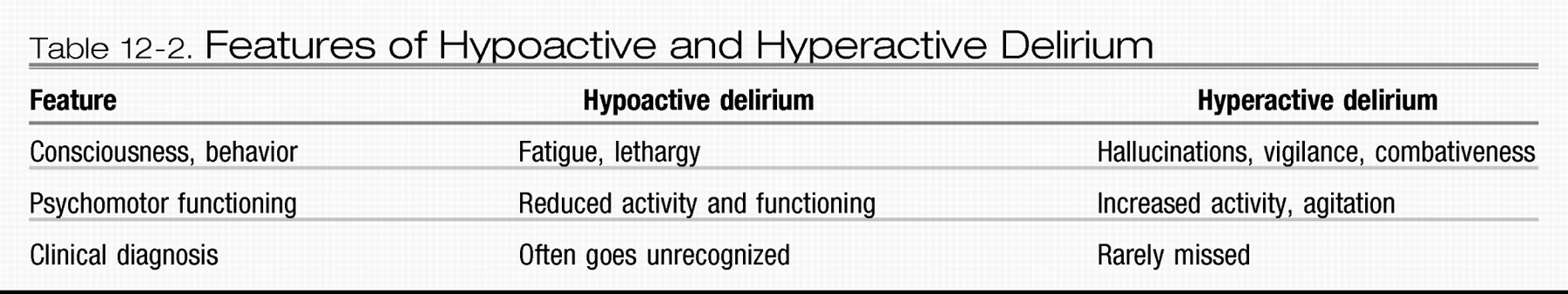
Clinically, delirium typically presents in one of two major forms: hypoactive or hyperactive (
Table 12-2). The hypoactive form, which is more common in older patients, is characterized by lethargy and reduced psychomotor functioning. It is important to note that the hypoactive form of delirium is associated with an overall poorer prognosis and often goes unrecognized by clinicians and caregivers (
Liptzin and Levkoff 1992;
Sandberg et al. 1999). The reduced level of patient activity associated with hypoactive delirium is often attributed to low mood or fatigue, which may contribute to its misdiagnosis or underrecognition. The hyperactive form of delirium is characterized by agitation, increased vigilance, and often concomitant hallucinations. The hyperactive form rarely goes unnoticed by caregivers or clinicians. Clinicians should be aware of a mixed form of delirium, in which patients fluctuate between the hypoactive and the hyperactive forms. The mixed form creates a challenge in distinguishing symptoms of delirium from symptoms of other psychotic or mood disorders.
PATHOPHYSIOLOGY OF DELIRIUM
The fundamental pathophysiological mechanisms of delirium remain unclear, most likely because many different etiologies may result in delirium through different mechanisms or pathways (
Flacker and Lipsitz 1999). Historically, delirium was thought to result from a functional rather than a structural lesion; electroencephalographic findings demonstrated global functional impairments and generalized slowing of alpha wave activity (
Pro and Wells 1977). Several studies of cerebral blood flow using positron emission tomography (PET) or single-photon emission computed tomography (SPECT) have found that delirium is associated mostly with decreased blood flow, especially in the prefrontal cortex, thalamus, basal ganglia, temporo-parietal cortex, and lingual gyri (
Burns et al. 2004;
Fong et al. 2006;
Trzepacz and van der Mast 2002). However, results from previous imaging studies have been highly variable.
Other neuroimaging studies, using either computed tomography (CT) or magnetic resonance imaging (MRI), have demonstrated structural abnormalities in the brains of patients with delirium, especially in the splenium of the corpus callosum, thalamus, and right temporal lobe (
Bogousslavsky et al. 1988;
Doherty et al. 2005;
Naughton et al. 1997;
Ogasawara et al. 2005;
Takanashi et al. 2006). Results from neuropsychological testing also suggest that delirium is related to disruptions in higher cortical function, especially in the frontal lobe region (
Rudolph et al. 2006). The leading current hypotheses view delirium as the final common pathway of many different pathogenic mechanisms, including imbalances in neurotransmission, inflammation, and chronic stress.
The most frequently considered mechanism of delirium is dysfunction in the cholinergic system. Acetylcholine plays a key role in mediating consciousness and attentional process. Given that delirium is manifested by an acute confusional state, often with alterations of consciousness, it is likely to have a cholinergic basis. Evidence for the cholinergic connection includes findings that anticholinergic drugs can induce delirium in humans and animals and that serum anticholinergic activity is increased in patients with delirium (
Marcantonio et al. 2006). Also, cholinesterase inhibitors have been found to reduce symptoms of delirium in some studies (
Gleason 2003;
Wengel et al. 1998). An excess of dopaminergic neurotransmitters has also been cited as a mechanism of delirium and is most likely related to the role they play in regulating the release of acetylcholine (
Trzepacz and van der Mast 2002). Elevated serotonin, such as that seen in hepatic encephalopathy and “serotonin syndrome,” is another proposed mechanism of delirium (
Marcantonio et al. 2006).
Other neurotransmitters, including norepinephrine, glutamate, and melatonin, have also been implicated in the development of delirium, most likely due to their interactions with cholinergic and dopaminergic pathways; however, support for their involvement is less substantiated (
Cole 2004;
Inouye 2006). Chronic stress induced by severe illness, trauma, or surgery involves sympathetic and immune system activation that may lead to delirium; this activation may include increased activity of the hypothalamic-pituitary-adrenal axis with hypercortisolism, release of cerebral cytokines that alter neurotransmitter systems, alterations in the thyroid axis, and modification of blood-brain barrier permeability.
NEUROPSYCHOLOGICAL ASSESSMENT OF DELIRIUM
Administering neuropsychological tests during an acute delirium phase can prove to be difficult, due to the patient's alteration in basic attention capabilities, and may provide minimal useful information beyond that obtained from global cognitive screening tools such as the Mini-Mental State Examination (
Folstein et al. 1975) followed by the CAM. Given that the hallmark of delirium is alteration in attention, additional neuropsychological instruments that target attention should be used when assessing delirium if possible.
Deficits in attention can be measured using tasks such as Digit Span forward and backward (
Wechsler 1989); backward reciting of the days of the week or the months of the year; Trail Making Test A (
Reitan 1958); and the visual search and attention task, which is a cancellation task that requires the patient to cross out letters and symbols that are identical to a target (
Trenerry et al. 1990). In one previous study, postoperative delirium was found to be associated with preoperative deficits in attention and executive function (
Rudolph et al. 2006), both of which are useful to evaluate in delirium. Executive function measures that are both brief and informative include the following: Trail Making Test B (
Reitan 1958), Digit Symbol Modalities Test (Wechsler 1997), Stroop Neuropsychological Screening Test (
Trenerry et al. 1989), and Controlled Oral Word Association Test (
Benton et al. 1983). A study of the Clock Drawing Test found that although this instrument was a good detector of overall cognitive impairment, it is not suitable for detection of delirium (
Adamis et al. 2005). Identification of cognitive measures that predict delirium onset has received some attention in the literature. For example, a study of the Mini-Mental State Examination demonstrated that four items (i.e., year, date, “sword” spelled backwards, and design copying) of the original 20 items are accurate screening measures for delirium (
Fayers et al. 2005).
RISK FACTORS FOR DELIRIUM
Delirium usually occurs as a result of multifactorial causes (
Inouye and Charpentier 1996). Although it can be caused by a single factor, delirium more typically develops due to the interrelationship between patient vulnerability at hospital admission and noxious insults or precipitating factors occurring during the course of hospitalization. For example, a single dose of a sedative given to a patient who is cognitively impaired or severely ill may lead to delirium. However, a patient without severe illness or cognitive impairment has greater resistance to developing delirium unless he or she is repeatedly exposed to multiple insults such as surgery, anesthesia, and psychoactive medications (
Gleason 2003). The observant clinician will recognize that addressing only a single noxious insult or factor may not aid in improving delirium but that all predisposing and precipitating factors need to be addressed for resolution of delirium.
Existing cognitive impairment and dementia are the leading risk factors for the development of delirium. In fact, patients with dementia have a two- to fivefold increased risk for developing delirium, and nearly two-thirds of cases of delirium occur in patients with dementia (
Cole 2004;
Inouye 2006;
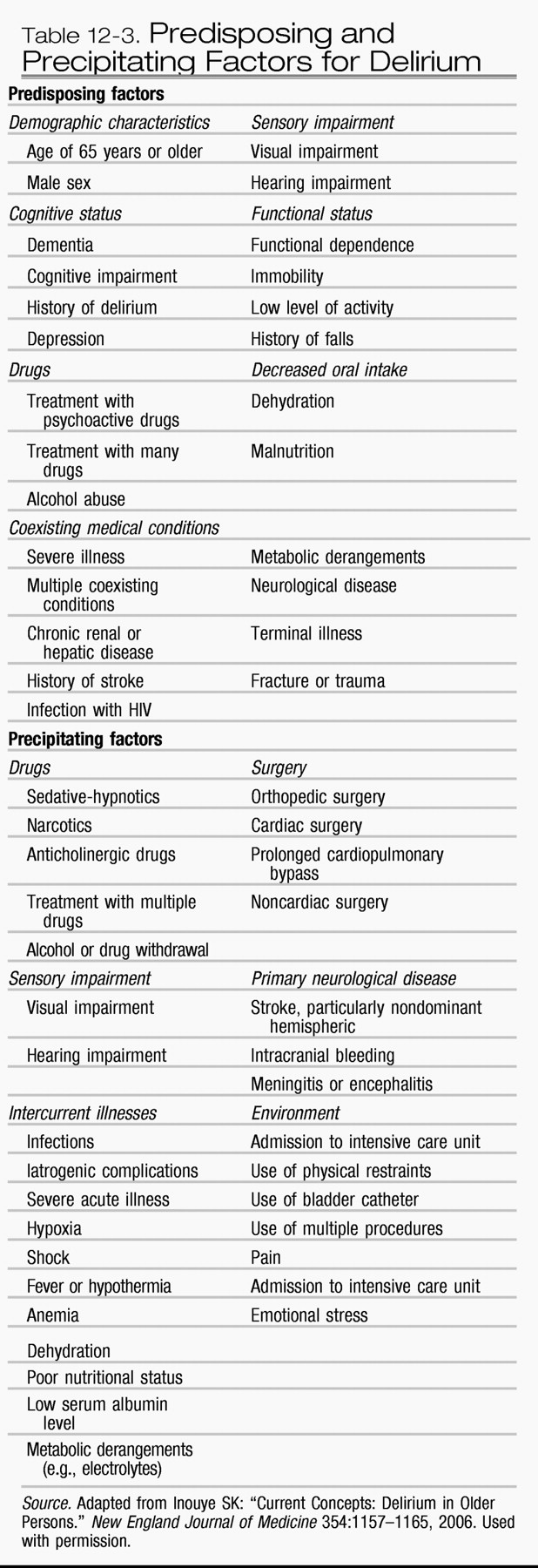
Trzepacz and van der Mast 2002). Other predisposing factors include advanced age, chronic or severe underlying illness, number and severity of comorbid conditions, functional impairment, male gender, dehydration, vision or hearing impairments, chronic renal insufficiency, history of alcohol abuse or dependence, and malnutrition (see
Table 12-3)(
Elie et al. 1998;
Francis 1992;
Rockwood 1989;
Rogers et al. 1989).
Various chronic medical illnesses also serve as predisposing factors to delirium, including neurological disorders (e.g., Parkinson's disease, cerebrovascular disease, mass lesions, trauma, infection, collagen vascular disease); systemic or nonneurological infections; metabolic alterations; and cardiac, pulmonary, endocrine, renal, and neoplastic conditions. A validated predictive model for development of delirium (
Inouye et al. 1993) at the time of hospital admission identified several independent risk factors, including severe underlying illness, vision impairment, baseline cognitive impairment, and a high blood urea nitrogen to creatinine ratio (used as an index of dehydration).
Predictive risk models that identify predisposing factors for delirium have been developed in specific medical populations, such as surgical patients, cancer patients, and nursing home patients (
Boyle 2006;
Hamann et al. 2005). A validated model for prediction of persistent delirium in hospitalized older patients at discharge has identified five risk factors: dementia, vision impairment, functional impairment, high comorbidity, and use of physical restraints during delirium (
Inouye et al. 2007). Overall, the development of these risk models aids in understanding the contribution of patient characteristics to delirium risk.
Precipitating factors for delirium include medications, immobilization, use of indwelling bladder catheters, use of physical restraints, dehydration, malnutrition, iatrogenic events, medical illnesses, organ insufficiency or failure (particularly renal or hepatic), infections, electrolyte or metabolic derangement, alcohol or drug intoxication or withdrawal, environmental influences, and psychosocial factors (see
Table 12-3) (
Agostini and Inouye 2003;
Inouye 2006). Decreased mobility, including that associated with the use of medical devices (e.g., indwelling bladder catheters and physical restraints), greatly increases the risk of delirium and functional decline (
Lazarus et al. 1991). Environmental factors (e.g., inadequate lighting, increased noise levels), psychosocial factors (e.g., depression, pain, anxiety), and iatrogenic events (e.g., transfusion reactions, allergic reactions) can also precipitate delirium.
With the introduction of oxygen-saturation monitoring (and subsequent decline of arterial blood gas determination), occult respiratory failure has emerged as an increasing problem for the development of delirium in elderly patients. Acute myocardial infarction and congestive heart failure commonly present with delirium as well. Metabolic disorders, such as hyper- or hyponatremia, hyper- or hypoglycemia, hypercalcemia, and thyroid or adrenal dysfunction, may also contribute to the development of delirium. Occult infection due to a variety of medical conditions (e.g., pneumonia, urinary tract infection, abdominal abscess) is a precipitating cause of delirium that is worth noting because older patients may not present with leukocytosis or the typical febrile response. A validated model of precipitating factors for the development of delirium in hospitalized older patients includes five factors: the use of physical restraints, malnutrition, more than three medications added during the previous day (over 70% of these were psychoactive drugs), indwelling bladder catheter, and any iatrogenic event (
Inouye and Charpentier 1996).
MEDICATIONS AND DELIRIUM
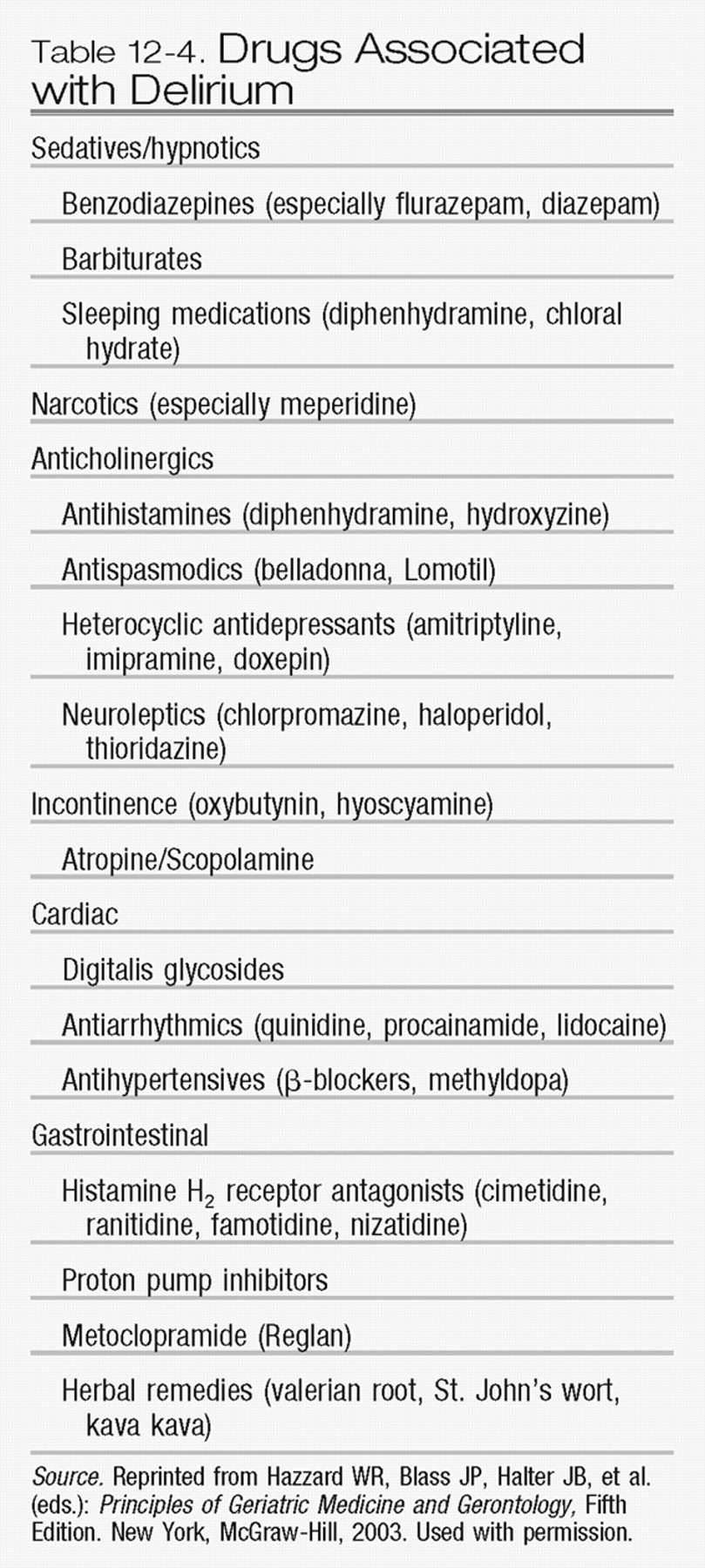
The role of medications in the development of delirium deserves special attention (
Table 12-4). Medication use contributes to delirium in more than 40% of cases (
Inouye 1994;
Inouye and Charpentier 1996). The medications most frequently associated with delirium are those with psychoactive effects, such as sedative-hypnotics, anxiolytics, narcotics, and histamine H
2 blockers. Drugs with anticholinergic effects, including antipsychotics, antihistamines, antidepressants, anti-parkinsonian agents, and anticonvulsants, are also commonly associated with delirium. Previous studies have demonstrated that the use of psychoactive medication results in a 4-fold increased risk of delirium, whereas the use of two or more psychoactive medications is associated with a 5-fold increased risk (
Inouye and Charpentier 1996). Sedative-hypnotic drugs are associated with a 3- to 12-fold increased risk of delirium, narcotics with a 3-fold risk, and anticholinergic drugs with a 5- to 12-fold risk (
Agostini and Inouye 2003;
Foy et al. 1995;
Inouye 2006;
Marcantonio et al. 1994;
Schor et al. 1992).
A greater number of medications prescribed leads to a proportionately greater increased risk for developing delirium. This is related to the direct toxicity of the medications themselves, as well as the increased risk of drug-drug and drug-disease interactions. Previous studies suggest that overuse of psychoactive drugs and poor management of medications commonly occur in hospitalized geriatric patients, providing support for the preventable nature of many cases of delirium (
Bates et al. 1995;
Lindley et al. 1992). Some homeopathic or herbal therapies, especially those used for mood disorders (e.g., St. John's wort, kava kava), may increase the risk of delirium, especially when used in combination with prescribed psychoactive medications.
Given the role of medications in contributing to the development of delirium, it is essential to conduct a complete review of all prescription and over-the-counter medications a patient is taking. The majority of older patients take several prescribed medications during hospitalization, increasing the risk for drug-drug and disease-drug interactions. Medications with known psychoactive effects should be discontinued or minimized whenever possible. At the very least, steps should be taken to reduce dosage or to substitute medications with less toxic potential. In aging adults, medications may cause adverse effects even when given at the recommended dosages and with serum drug levels that are within the “therapeutic range.” Determining if the patient has a history of chronic medication use or alcohol dependence is critical in assessing for withdrawal risk.
DIAGNOSIS AND DIFFERENTIAL DIAGNOSIS OF DELIRIUM
The diagnosis of delirium is based on clinical observation and relies on a thorough cognitive assessment, a detailed history from an informant close to the patient, and a comprehensive physical and neurological examination. The goal of a thorough background history interview is to establish that a change in cognition has occurred from the patient's baseline functioning. Acute alterations in cognition, representing abrupt deteriorations in mental status, may occur over hours or weeks, although it is important to keep in mind that these alterations may be superimposed on an underlying dementia as well. Delirium goes unrecognized by clinicians in up to 70% of patients who develop this condition (
Rockwood et al. 1994); therefore, careful clinical assessment for this condition is imperative. To facilitate immediate and effective diagnosis and treatment of delirium, it is important to identify all multifactorial contributors. Identification of these factors relies on insightful clinical judgment combined with a thorough medical evaluation. Guidelines have been established by the
American Psychiatric Association (1999) and the
Royal College of Physicians (2006), both of which outline approaches to diagnosis and management of delirium in the older population.
The initial step during evaluation for delirium should be to determine the extent of change from the patient's baseline cognitive status by a careful history with a reliable proxy informant. The importance of obtaining a complete history that focuses on the patient's baseline cognitive status and chronology of recent mental status changes cannot be underestimated. The clinician should also assess recent changes or updates in medication regimen, new infections, or recent development of medical illnesses that may contribute to delirium.
The next step involves conducting a careful assessment of the patient's cognitive status (see “Neuropsychological Assessment of Delirium” section above). Assessment of vital signs often aids in identifying factors such as fever, tachycardia, or tachypnea, each of which may provide important etiological clues. Physical examination for signs of medical illnesses or occult infections such as pneumonia, urinary tract infection, or acute abdominal processes should also be conducted. Often, delirium may be the initial and only sign of a serious and life-threatening underlying illness, such as sepsis, pneumonia, or myocardial infarction. Additionally, a detailed neurological examination assessing for focal changes or evidence of head trauma, infection, or cerebrovascular disease should be performed during the evaluation.
The clinician's most important and difficult task is to differentiate delirium from dementia. Traditionally, delirium has been conceptualized as a brief and transient condition; however, many recent studies have shown that delirium symptoms may persist for months or years (
Levkoff et al. 1994;
Marcantonio et al. 2003;
McCusker et al. 2003). Previous studies have indicated that dementia is the leading risk factor for delirium and that nearly two-thirds of cases of delirium occur in patients with dementia (
Cole 2004;
Inouye 2006). Patients with dementia who develop a superimposed delirium experience a more rapid progression of cognitive dysfunction and worse long-term prognosis (
Fick and Foreman 2000;
Jackson et al. 2004).
The key diagnostic feature that aids in distinguishing these two conditions is that delirium has an acute and rapid onset, whereas dementia is much more gradual in progression. Alterations in attention and changes in level of consciousness also point to a diagnosis of delirium. However, establishing the occurrence of those changes can be difficult in the face of missing baseline cognitive data or if preexisting cognitive deficits are reported by an informant. If the differentiation cannot be made with certainty, then given the life-threatening nature of delirium, the patient should be treated as delirious until proven otherwise.
Other important diagnoses that must be differentiated from delirium include psychiatric conditions such as depression, mania, and nonorganic psychotic disorders including schizophrenia. In general, these conditions do not develop suddenly in the context of a medical illness. Although hallucinations and perceptual disturbances can occur within the context of delirium, alterations in attention and global cognitive impairment are the key features that help to identify delirium. Differentiating among diagnoses is critical because delirium carries a more serious prognosis without proper evaluation and management. For example, treatment for certain conditions such as depression or affective disorders may involve the use of drugs with anticholinergic activity, which in turn could exacerbate an unrecognized case of delirium. Establishing the diagnosis can be difficult when the clinician is faced with symptoms that are subtle, when a background history is unavailable, or when the clinician is faced with an uncooperative patient. Again, given the seriousness of delirium and the fact that certain medical treatments may actually worsen symptoms, it is best for the clinician to assume that delirium is present until further diagnostic information is available.
No specific laboratory tests currently exist that will aid in the definitive identification of delirium. The laboratory evaluation for delirium is intended to identify contributing factors that will need to be addressed, and the approach should be guided by astute clinical judgment and tailored to the individual situation. Laboratory tests that should be considered in the delirium evaluation include complete blood count, electrolytes, kidney and liver function, oxygen saturation, and glucose levels. Evaluation of occult infection can be obtained through blood cultures, urinalysis, and urine culture. Other laboratory tests, such as thyroid function, arterial blood gas, vitamin B12 level, cortisol level, drug levels, toxicology screen, and ammonia levels, are also helpful in identifying factors that contribute to delirium. An electrocardiogram and/or chest radiograph may prove useful in patients with cardiac or respiratory diseases.
Brain imaging, using CT, PET, SPECT, or MRI, is indicated in the cases of head trauma or injury, evaluation of new focal neurological symptoms, evaluation for suspected encephalitis, or development of fever of unknown origin. Electroencephalography serves a limited role in the diagnosis of delirium—it has a false-negative rate of 17% and a false-positive rate of 22% (
Pisani et al. 2003;
Trzepacz et al. 1988)—and is most useful for detecting an occult seizure disorder. Cerebrospinal fluid examination, accomplished through lumbar puncture, may be useful in cases of febrile delirium or to exclude meningitis or encephalitis. Overall, the routine use of neuroimaging in delirium is not recommended, because the overall diagnostic yield is low, and the findings from neuroimaging change the management of patients in less than 10% of cases (
Hirao et al. 2006).