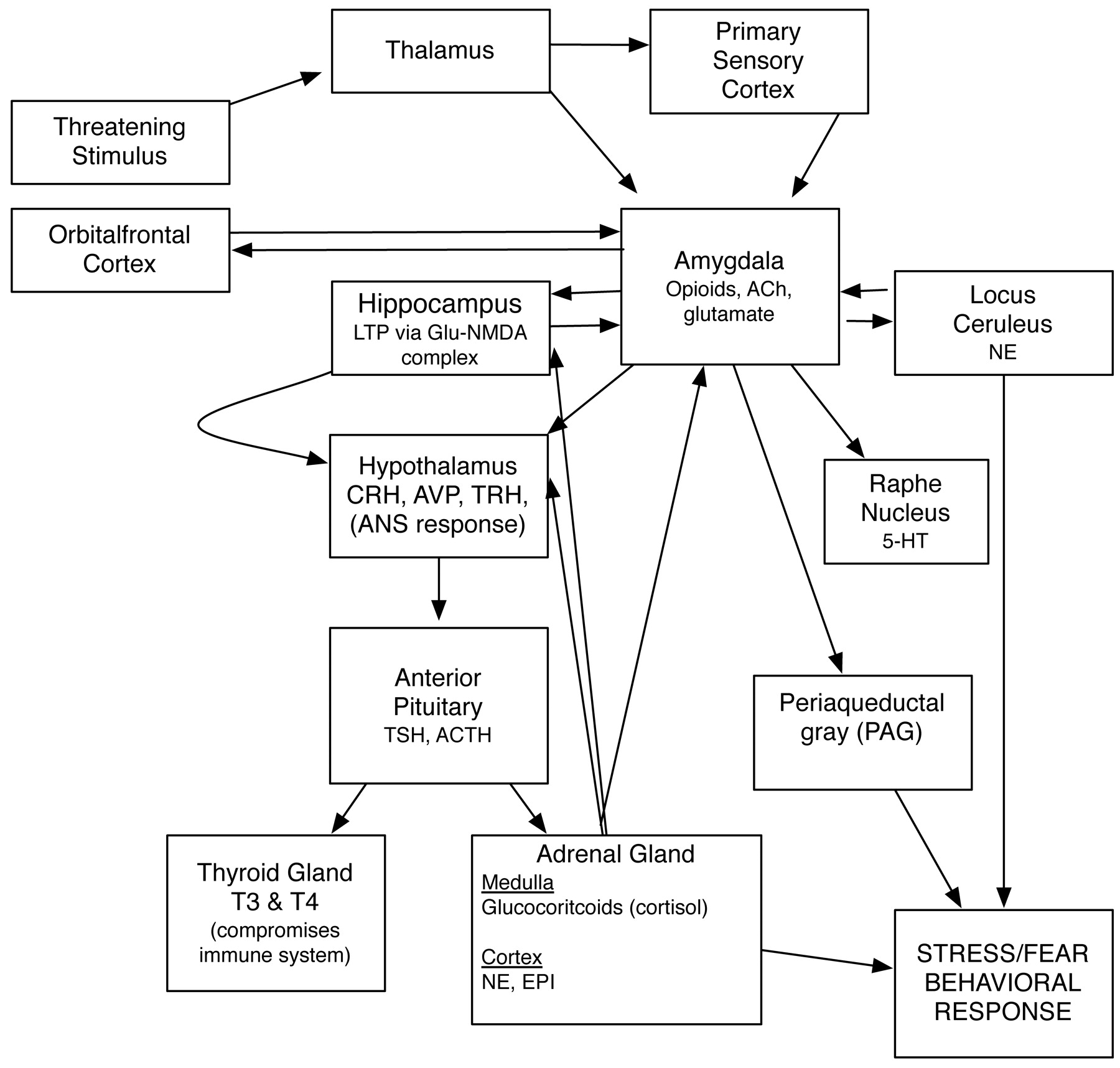
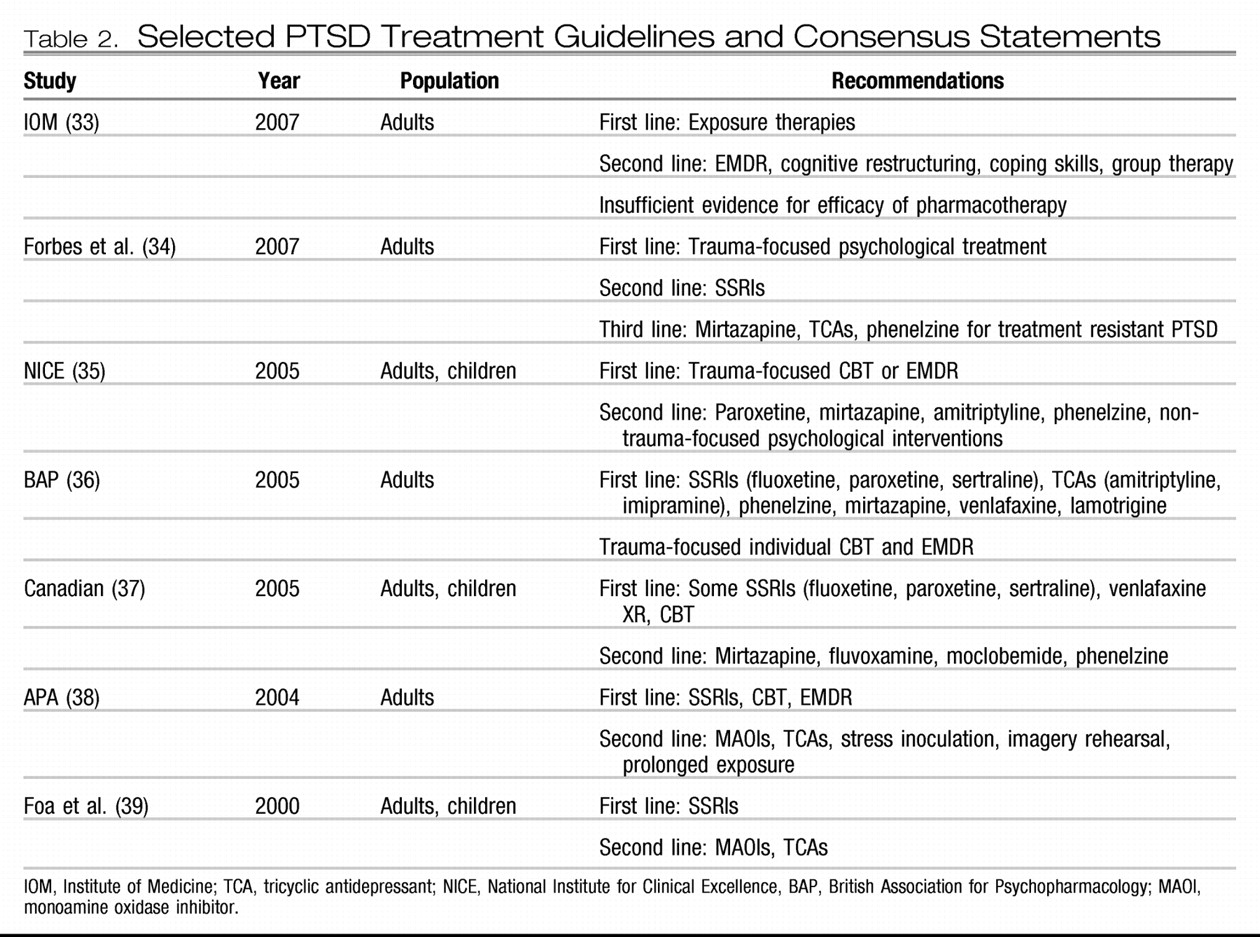
EXPOSURE-BASED CBTS
Trials of exposure-based CBTs have generally included components of psychoeducation, breathing, and relaxation training. By definition, these exposure therapies also incorporate some form of reexposure to past traumatic experience (e.g., imaginal, in vivo, written, verbal, or taped narrative recounting) into sessions. In addition, homework is often included.
In 2006, Monson et al. (
41) reported the results of a wait-list-controlled study of cognitive processing therapy in 60 combat veterans. The overall dropout rate was 16.6% (20% from cognitive processing therapy and 13% from the wait-list), but analyses of the intention-to-treat sample revealed significant improvements in both PTSD and co-occurring depressive symptoms in the treatment group compared with the wait-list group.
The effectiveness of cognitive processing therapy was also demonstrated in a study reported in 2005 by Chard (
42) of 71 adult sexual abuse survivors with PTSD. The control was a minimal-attention wait-list group. Participants were assessed before treatment, immediately after treatment, 3 months after treatment, and 1 year after treatment using the Clinician Administered PTSD Scale (CAPS), considered a “standard” for assessing PTSD symptoms and severity in research settings, and several other rating scales. Analysis demonstrated that cognitive processing therapy was superior to wait-list in reducing PTSD symptoms and that reductions were maintained for at least 1 year.
Resick et al. (
43) dismantled the components of cognitive processing therapy recently. They randomly assigned 150 adult women with PTSD to three conditions: 1) full cognitive processing therapy, which included both exposure (i.e., writing and reading a detailed account of the trauma) and cognitive therapy (i.e., challenging patient assertions about the meaning of the trauma and the implications for the patient's life); 2) cognitive therapy without the writing and reading component; and 3) the writing and reading component without cognitive therapy. All conditions included 2 hours of therapy per week for 6 weeks. Blinded raters assessed all participants for PTSD (using CAPS) and depression weekly during the treatment, at 2 weeks after the last session of therapy, and at 6 months. Although all treatment completers still met the criteria for PTSD at the conclusion of the study, substantial improvement was observed in all three treatment groups on primary PTSD and depression outcomes and on secondary measures of anxiety, guilt, and shame. Cognitive therapy without exposure was associated with the greatest improvement. This result suggested that the cognitive component of this therapy (i.e., altering the meaning of the traumatic event) may occur without repeated and explicitly evoked fear memories. It also suggested that cognitive processing therapy might be characterized as more cognitive than exposure-based therapy.
In 2007, Schnurr et al. (
44) reported the results of prolonged exposure for female veterans (N=277) and active duty personnel (N=7) across 12 military and veterans affairs centers specializing in medical treatment for military veterans. Patients were randomly assigned to receive prolonged exposure therapy (N=141) or present-centered therapy (N=143) delivered in 10 weekly 90-minute sessions, and outcomes were assessed before and immediately after treatment and again at 3 and 6 months after treatment. Immediately after treatment, the prolonged exposure group was more likely than the present-centered therapy group to no longer meet PTSD criteria (41% compared with 27.8%, odds ratio [OR]=1.80, confidence interval [CI]=95%) and more likely to achieve full remission (15.2% compared with 6.9%, OR=2.43, CI=95%). These results were maintained at the 3- and 6-month follow-up. Although this was a study of military personnel and veterans, 70% of participants indicated sexual trauma as their index (worst) traumatic experience, Seventeen percent more participants dropped out of the prolonged exposure arm than out of the present-centered arm.
A controlled study reported in 2005 by Rothbaum et al. (
45) evaluated the relative efficacy of prolonged exposure therapy and eye movement desensitization and reprocessing (EMDR) (see below). In this study, 74 adult female rape victims (index rape occurring either in adulthood or childhood) were randomly assigned to nine-session prolonged exposure, EMDR, and wait-list control groups. Dropout rates across the groups were not significantly different. Immediately after treatment, the groups receiving prolonged exposure and EMDR both demonstrated statistically significant improvement across three outcome measures, including a 50% or more decrease from baseline in CAPS score (p=0.001). After treatment, 95% of participants who received prolonged exposure therapy and 75% of participants who received EMDR no longer met the criteria for PTSD. Individuals who received both treatments showed significantly reduced depressive symptoms and dissociative symptoms immediately and at 6 months. Results were maintained at the 6-month follow-up for the prolonged exposure group across PTSD, depressive, and dissociative symptoms but were maintained to a significantly lesser extent for the EMDR group with regard to PTSD.
The effectiveness of brief exposure therapy has been demonstrated in two recent studies reported in 2005 and 2007 by Basoglu et al. (
46,
47). In the first study, 59 earthquake survivors with PTSD assessed by CAPS were randomly assigned to a single-session exposure-based behavioral therapy intervention (in which the intensity of simulated trauma was adjusted in accordance with the patient's personal feelings of comfort) or to a wait-list (
46). At 6, 12, and 24 weeks after treatment, as well as at 1–2 years after treatment, the treatment group was observed to have significant decreases in CAPS score, Beck Depression Inventory score, and other patient self-measures of fear and anxiety, and of overall impression. With regard to CAPS, effect sizes were considerable (Cohen's
d=0.7–1.4), and the improvement rate rose from 49% at week 6 to more than 80% at other assessment points. In the second study (
47), 31 earthquake survivors with PTSD were randomly assigned to a single-session exposure-based behavioral therapy (N=16) or to repeated assessments (N=15). Participants were assessed at 4, 8, 12, and 24 weeks after treatment and after 1–2 years. Again, significant between-group treatment effects were observed in PTSD (assessed by CAPS) and assessor-rated global improvement (Global Improvement Scale–Assessor), with significant between-group treatment effects observed in both outcome measures at week 8. Improvement rates of 40% at week 4 rose to 80% by week 24 and at the 1–2 year follow-up, with large effect sizes (Cohen's
d=0.9–1.7) noted across primary measures at week 8.
EMDR
EMDR continues to be examined as a treatment for victims of trauma; however, many of the studies published since 2004 include participants without a formal diagnosis of PTSD. An exception is a study reported in 2007 by van der Kolk et al. (
48), in which 88 patients with PTSD were randomly assigned to 8 weeks of EMDR, fluoxetine, or placebo. Symptoms were assessed using the CAPS and Beck Depression Inventory II immediately after treatment and at 6 months. At the 6-month follow-up, 75% of the patients with adult-onset PTSD (compared with 33% of those with childhood-onset PTSD) receiving EMDR achieved remission compared with none of the patients receiving fluoxetine. Neither treatment produced complete symptom remission in the majority of the patients with childhood-onset PTSD. It should be noted that fluoxetine was discontinued at termination of the 8-week treatment phase, so the poor SSRI outcomes at 6 months should not be surprising.
In a study reported in 2007, Högberg et al. (
49) examined 24 transportation workers who had either been assaulted or who had witnessed a person-under-train accident and who met DSM-IV-TR criteria for PTSD. Participants were randomly assigned to either five sessions of EMDR or to a wait-list. After treatment, 8 of 13 patients receiving EMDR (67%) no longer met the criteria for PTSD compared with 1 of 11 (11%) patients on the wait-list (p=0.02). Significant differences were also observed in Global Assessment of Functioning and Hamilton Depression Rating Scores (HAM-D) scores.
Neither of these studies dismantled the effects of exposure compared with eye-movement components of the treatment. Previous studies have shown that the eye movements are not critical to the treatment effect. These small studies suggest efficacy of brief EMDR in sexual assault victims and witnesses to vehicular accidents but cannot be generalized to combat veterans.
OTHER PSYCHOTHERAPIES
Studies of other types of psychotherapy, including coping skills therapy, eclectic psychotherapy, psychodynamic psychotherapy, cognitive restructuring, and brainwave neurofeedback, have also been published in recent years, but the utility and generalizability of conclusions from these studies are limited by methodological issues such as lack of formalized diagnostic procedures, inclusion of patients who did not have PTSD, very high dropout rates, unspecified handling of dropouts or missing data, and use of nonblinded assessors. A study reported in 2004 by Neuner et al. (
50) of coping skills therapy in 43 war refugees, although methodologically sound, failed to demonstrate a differential effect of treatment.
Case reports (
51,
52) have recently suggested that exposure-based therapy may be facilitated through the use of computerized audiovisual simulations of a traumatic combat environment. The effectiveness of this facilitated CBT—termed “virtual reality therapy”—in disaster workers with PTSD has also been demonstrated in a small controlled trial. In 2007, Difede et al. (
53) assigned 21 September 11 terrorist attack workers to either virtual reality treatment (N=13) or wait-list control (N=8). The treatment group showed a significant decline in CAPS scores compared with the wait-list group. Although these reports are encouraging, larger randomized controlled trails must replicate such findings before virtual reality therapy can be recommended with high confidence.