CLINICAL CONSIDERATIONS AND RECOMMENDATIONS
▶ What is the evidence regarding the safety and efficacy of treatment for depression during pregnancy?
Most data related to antidepressants in pregnancy are derived from the use of selective serotonin reuptake inhibitors (SSRIs) (fluoxetine, sertraline, citalopram, and paroxetine). Overall, there is limited evidence of teratogenic effects from the use of antidepressants in pregnancy or adverse effects from exposure during breastfeeding (
62–
64). There are two reports from GlaxoSmithKline based on a Swedish national registry and a U.S. insurance claims database that have raised concerns about a 1.5–2-fold increased risk of congenital cardiac malformations (atrial and ventricular septal defects) associated with first-trimester paroxetine exposure (
www.gskus.com/news/paroxetine/paxil_letter_e3.pdf). The manufacturer subsequently changed paroxetine's pregnancy FDA category from C to D (
www.fda.gov/cder/drug/advisory/paroxetine200512.htm).
More recently, the teratogenic effect of SSRI use during the first trimester of pregnancy was examined in two large case-control studies from multisite surveillance programs (
65,
66). In the National Birth Defects Prevention Study, no significant associations were found between SSRI use overall and congenital heart defects (
66). However, an association was found between SSRI use (particularly paroxetine) during early pregnancy and anencephaly, craniosynostosis, and omphalocele. Importantly, these risks were found only after more than 40 statistical tests were performed. Even if findings were not the result of chance, the absolute risks associated with SSRI use identified in this study were small. For example, a twofold to threefold increase in birth defects would occur for omphalocele (1 in 5,000 births), craniosynostosis (1 in 1,800 births) and anencephaly (1 in 1,000 births). In contrast, in the Slone Epidemiology Center Birth Defects Study no increased risk of craniosynostosis, omphalocele, or heart defects associated with SSRI use overall during early pregnancy was found (
65). An association was seen between paroxetine and right ventricular outflow defects. Additionally, sertraline use was associated with omphalocele and atrial and ventricular septum defects. A limitation of this study is that the authors conducted 42 comparisons in their analyses for their main hypotheses. Both of these case-control studies were limited by the small number of exposed infants for each individual malformation. The current data on SSRI exposure during early pregnancy provide conflicting data on the risk for both overall and specific malformations. Some investigators have found a small increased risk of cardiac defects, specifically with paroxetine exposure. The absolute risk is small and generally not greater than two per 1,000 births; hence, these agents are not considered major teratogens.
Exposure to SSRIs late in pregnancy has been associated with transient neonatal complications, including jitteriness, mild respiratory distress, transient tachypnea of the newborn, weak cry, poor tone, and neonatal intensive care unit admission (
67–
71). A more recent FDA public health advisory highlighted concerns about the risk of an unconfirmed association of newborn persistent pulmonary hypertension with SSRI use (
72) (
www.fda.gov/cder/drug/advisory/SSRI_PPHN200607.htm).
The potential risk of SSRI use in pregnancy must be considered in the context of the risk of relapse of depression if treatment is discontinued. Factors associated with relapse during pregnancy include a long history of depressive illness (more than 5 years) and a history of recurrent relapses (more than four episodes) (
13). Therefore, treatment with all SSRIs or selective norepinephrine reuptake inhibitors or both during pregnancy should be individualized. At this time, paroxetine use in pregnant women and women planning pregnancy should be avoided, if possible. Fetal echocardiography should be considered for women exposed to paroxetine in early pregnancy. Because abrupt discontinuation of paroxetine has been associated with withdrawal symptoms, discontinuation of this agent should occur according to the product's prescribing information.
Tricyclic antidepressants (TCAs) have been available in the United States since 1963 and were widely used by women during pregnancy and lactation before the introduction of SSRIs. Results from initial studies, which suggested that TCA exposure might be associated with limb anomalies (
73–
75), have not been confirmed with subsequent studies (
76,
77). Neonatal neurobehavioral effects from fetal exposure have not been reported (
78).
Acute effects associated with TCA exposure include case reports of fetal tachycardia (
79), neonatal symptoms such as tachypnea, tachycardia, cyanosis, irritability, hypertonia, clonus, and spasm (
72–
82), and transient withdrawal symptoms (
83). In more recent studies, a significant link between prenatal exposure to TCAs and perinatal problems has not been documented (
64,
84–
86).
Atypical antidepressants are non-SSRI and non-TCA antidepressants that work by distinct pharmacodynamic mechanisms. The atypical antidepressants include bupropion, duloxetine, mirtazapine, nefazodone, and venlafaxine. The limited data of fetal exposure to these antidepressants (
70,
85–
89), do not suggest an increased risk of fetal anomalies or adverse pregnancy events. In the one published study of bupropion exposure in 136 patients, a significantly increased risk of spontaneous abortion, but not an increased risk of major malformations, was identified (
90). In contrast, the bupropion registry maintained at GlaxoSmithKline has not identified any increased risk of spontaneous abortion, although these data have not undergone peer review.
Antidepressant medication is the mainstay of treatment for depression, although considerable data show that structured psychotherapy, such as interpersonal psychotherapy or cognitive behavioral therapy, are effective treatments for mild to moderate depression and are beneficial adjuncts to medication. In addition, electroconvulsive therapy is an effective treatment for major depression and is safe to use during pregnancy (
91,
92).
▶ What is the evidence regarding the safety and efficacy of lithium for the treatment of bipolar disorders during pregnancy?
Use of lithium in pregnancy may be associated with a small increase in congenital cardiac malformations. The initial retrospective data suggested that fetal exposure to lithium was associated with a 400-fold increase in congenital heart disease, particularly Ebstein's anomaly (
93,
94). A subsequent meta-analysis of the available data calculated the risk ratio for cardiac malformations to be 1.2–7.7 and the risk ratio for overall congenital malformations to be 1.5–3 (
95). In more recent small studies, limited in their statistical power, the magnitude of early estimates of teratogenic potential of lithium could not be confirmed (
96–
98).
Fetal exposure to lithium later in gestation has been associated with fetal and neonatal cardiac arrhythmias (
99), hypoglycemia, nephrogenic diabetes insipidus (
100), polyhydramnios, reversible changes in thyroid function (
101), premature delivery, and floppy infant syndrome similar to that seen with benzodiazepine exposure (
102). Symptoms of neonatal lithium toxicity include flaccidity, lethargy, and poor suck reflexes, which may persist for more than 7 days (
103). Neurobehavioral sequelae were not documented in a 5-year follow-up of 60 school-aged children exposed to lithium during gestation (
104).
The physiologic alterations of pregnancy may affect the absorption, distribution, metabolism and elimination of lithium, and close monitoring of lithium levels during pregnancy and postpartum is recommended. The decision to discontinue lithium therapy in pregnancy because of fetal risks should be balanced against the maternal risks of exacerbation of illness. In a recent study, it was reported that abrupt discontinuation of lithium was associated with a high rate of bipolar relapse among pregnant women (
39). The following treatment guidelines have been suggested for women with bipolar illness who are treated with lithium and plan to conceive: 1) in women who experience mild and infrequent episodes of illness, treatment with lithium should be gradually tapered before conception; 2) in women who have more severe episodes but are only at moderate risk for relapse in the short term, treatment with lithium should be tapered before conception but reinstituted after organogenesis; 3) in women who have especially severe and frequent episodes of illness, treatment with lithium should be continued throughout gestation and the patient counseled regarding reproductive risks (
95). Fetal assessment with fetal echocardiography should be considered in pregnant women exposed to lithium in the first trimester. For women in whom an unplanned conception occurs while receiving lithium therapy, the decision to continue or discontinue the use of lithium should be in part based on disease severity, course of the patient's illness, and the point of gestation at the time of exposure.
▶ What is the evidence regarding the safety and efficacy of the antiepileptic drugs valproate and carbamazepine for the treatment of bipolar disorders during pregnancy?
Several anticonvulsants, including valproate, carbamazepine, and lamotrigine, currently are used in the treatment of bipolar disorder. Data regarding fetal effects of these drugs are derived primarily from studies of women with seizures. Whether the underlying pathology of epilepsy contributes to the teratogenic effect on the fetus is unclear. Epilepsy may not contribute to the teratogenic effects of antiepileptic drugs based on the results of a recent study that demonstrated similar rates of anomalies between infants of women without epilepsy and infants of women with epilepsy but who had not taken antiepileptic drugs during pregnancy (
105).
Prenatal exposure to valproate is associated with a 1–3.8% risk of neural tube defects, with a corresponding dose-response relationship (
106–
113). Other congenital malformations associated with valproate use include craniofacial anomalies (
114), limb abnormalities (
115), and cardiovascular anomalies (
116–
118). A “fetal valproate syndrome” has been described with features of fetal growth restriction, facial dysmorphology, and limb and heart defects (
119–
121). Varying degrees of cognitive impairment, including mental development delay (
122), autism (
123–
126), and Asperger's syndrome (
124), have been reported with fetal valproate syndrome (
124,
127,
128). Acute neonatal risks include hepatotoxicity (
129), coagulopathies (
130), neonatal hypoglycemia (
131), and withdrawal symptoms (
132).
Carbamazepine exposure in pregnancy is associated with a fetal carbamazepine syndrome manifest by facial dysmorphism and fingernail hypoplasia (
124,
133–
136). It is unclear whether carbamazepine use increases the risk of fetal neural tube defects or developmental delay (
124,
127,
133–
139). Fetal exposure to lamotrigine has not been documented to increase the risk of major fetal anomalies (
140–
145), although there may be an increased risk of midline facial clefts (0.89% of 564 exposures) as reported by one pregnancy registry (
143), possibly related to higher daily maternal doses (greater than 200 mg/day) (
145). The reproductive safety of lamotrigine appears to compare favorably with alternative treatments, but lacking are studies of the effectiveness of this antiepileptic drug as a mood stabilizer in pregnancy.
In managing bipolar disorders, the use of valproate and carbamazepine are superior to that of lithium for patients who experience mixed episodes or rapid cycling but exhibit limited efficacy in the treatment of bipolar depression. In contrast, lamotrigine is efficacious in the prevention of the depressed phase of illness (
146,
147). Lamotrigine is a potential maintenance therapy option for pregnant women with bipolar disorder because of its protective effects against bipolar depression, general tolerability, and growing reproductive safety profile relative to alternative mood stabilizers. Because both valproate and carbamazepine are associated with adverse effects when used during pregnancy, their use, if possible should be avoided especially during the first trimester. The effectiveness of folate supplementation in the prevention of drug-associated neural tube defects has not been documented; however, folate supplementation of 4 mg/day should be offered preconceptionally and for the first trimester of pregnancy. Prenatal surveillance for congenital anomalies by maternal serum alpha-fetoprotein level testing, fetal echocardiography, or a detailed ultrasound examination of the fetal anatomy or a combination of these procedures should be considered. Whether the use of antiepileptic drugs such as carbamazepine increase the risk of neonatal hemorrhage and whether maternal vitamin K supplementation is effective remains unclear (
148).
▶ What is the evidence regarding the safety and efficacy of treatment for anxiety disorders during pregnancy?
Use of benzodiazepines does not appear to carry a significant risk of somatic teratogenesis. In early studies of in utero exposure to diazepam, a benzodiazepine, an increased risk of oral clefts was reported (
149–
151). In a subsequent meta-analysis, it was demonstrated that prenatal benzodiazepine exposure increased the risk of oral cleft, although the absolute risk increased by 0.01%, from 6 in 10,000 to 7 in 10,000 (
76). In a recent case-control study of 22,865 infants with congenital anomalies and 38,151 infants without congenital anomalies, an association of congenital anomalies, including oral clefts with exposure to five different benzodiazepines, was not found (
152). Similar findings were documented in a case-control study of clonazepam (
153). If discontinuation of benzodiazepine use is considered during pregnancy, benzodiazepines should not be abruptly withdrawn.
The data regarding neonatal toxicity and withdrawal syndromes are well documented, and neonates should be observed closely in the postpartum period. Floppy infant syndrome, characterized by hypothermia, lethargy, poor respiratory effort, and feeding difficulties, is associated with maternal use of benzodiazepines shortly before delivery (
154–
162). Neonatal withdrawal syndromes, characterized by restlessness, hypertonia, hyperreflexia, tremulousness, apnea, diarrhea, and vomiting, have been described in infants whose mothers were taking alprazolam (
163), chlordiazepoxide (
164–
166), or diazepam (
167,
168). These symptoms have been reported to persist for as long as 3 months postpartum (
81).
The long-term neurobehavioral impact of prenatal benzodiazepine exposure is unclear. The existence of a “benzodiazepine-exposure syndrome,” including growth restriction, dysmorphism, and both mental and psychomotor retardation, in infants exposed prenatally to benzodiazepines is disputed (
169–
171). In one study, no differences in the incidence of behavioral abnormalities at age 8 months or IQ scores at age 4 years were found among children exposed to chlordiazepoxide during gestation (
172).
▶ What is the evidence regarding the safety and efficacy of treatment for schizophrenia during pregnancy?
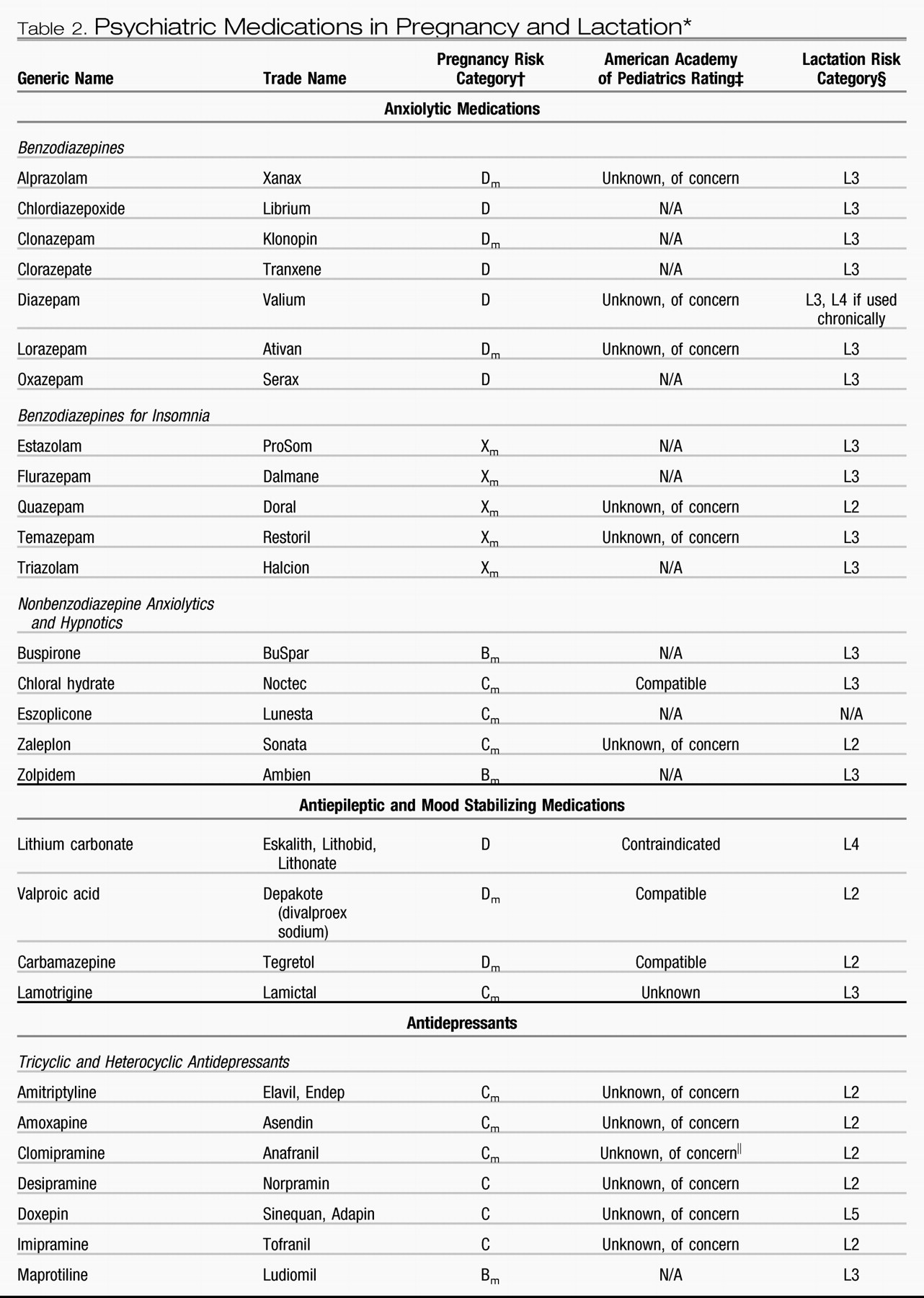
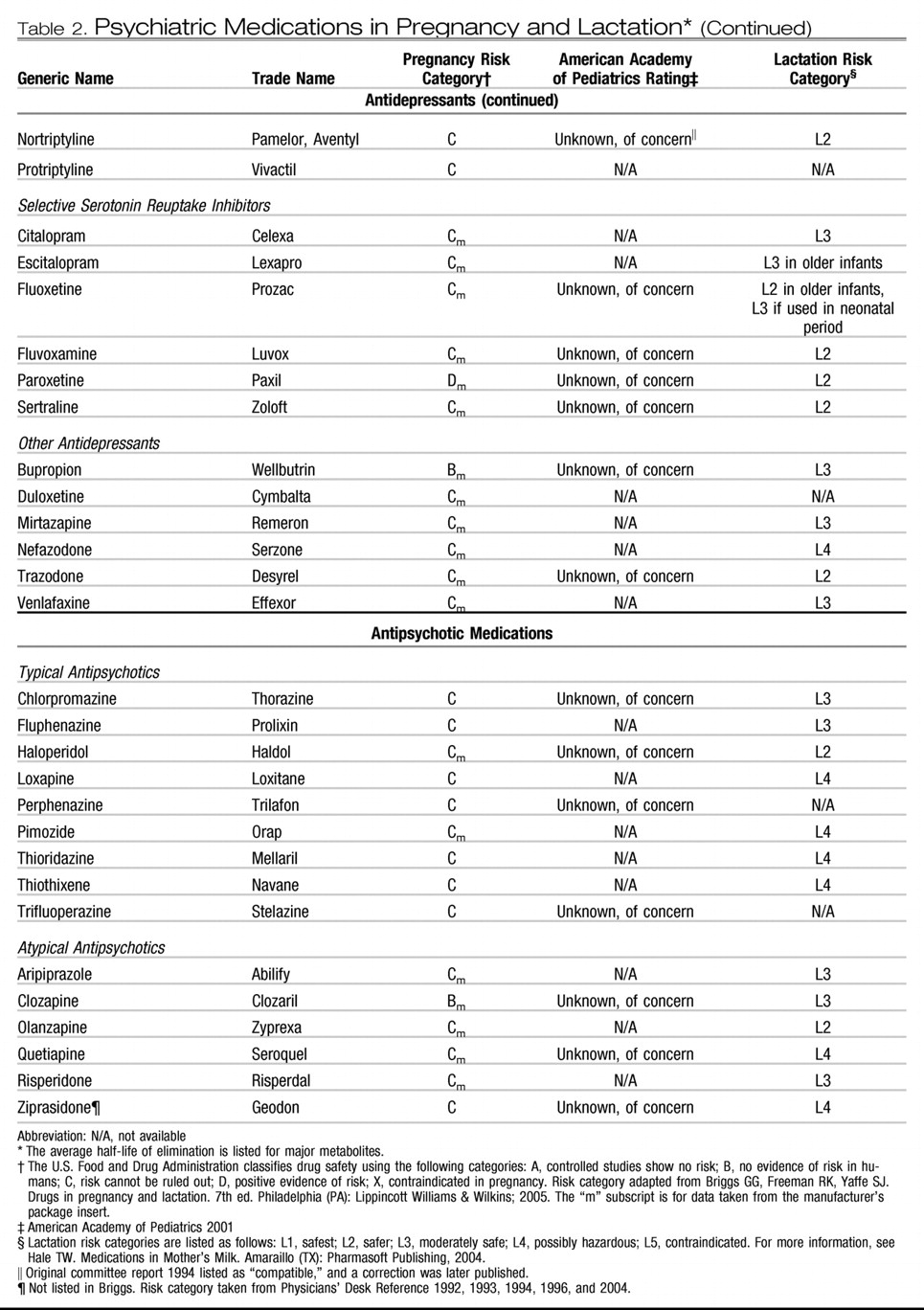
The atypical antipsychotics (eg, clozapine, olanzapine, quetiapine, risperidone, ziprasidone, and aripiprazole) have replaced the typical agents as first-line medications for psychotic disorders (
Table 2). The atypical antipsychotics generally are better tolerated and possibly are more effective in managing the negative symptoms of schizophrenia. They also are used increasingly for bipolar disorder, obsessive-compulsive disorder, and treatment-resistant depression. The reproductive safety data regarding the use of atypical antipsychotics remains extremely limited. In a prospective comparative study of pregnancy outcomes between groups exposed and unexposed to atypical antipsychotics, outcomes of 151 pregnancies with exposure to olanzapine, risperidone, quetiapine, and clozapine demonstrated a higher rate of low birth weight (10% in the exposed versus 2% in the nonexposed group) and therapeutic abortions (
173).
The typical antipsychotic drugs have a larger reproductive safety profile and include haloperidol, thioridazine, fluphenazine, perphenazine, chlorpromazine, and trifluoperazine. No significant teratogenic effect has been documented with chlorpromazine, haloperidol, and perphenazine (
174–
176). In a study of 100 women treated with haloperidol (mean dose of 1.2 mg/day) for hyperemesis gravidarum, no differences in gestational duration, fetal viability, or birth weight were noted (
177). In a large prospective study encompassing approximately 20,000 women treated primarily with phenothiazines for emesis (
178), investigators found no significant association with neonatal survival rates or severe anomalies. Similar results have been obtained in several retrospective studies of women treated with trifluoperazine for repeated abortions and emesis (
179,
180). In contrast, other investigators reported a significant association of major anomalies with prenatal exposure to phenothiazines with an aliphatic side chain but not with piperazine or piperidine class agents (
181). Reanalysis of previously reported data obtained also identified a significant risk of malformations associated with phenothiazine exposure in weeks 4–10 of gestation (
182). In clinical neurobehavioral outcome studies encompassing 203 children exposed to typical antipsychotics during gestation, no considerable differences have been detected in IQ scores at 4 years of age (
183,
184), although relatively low antipsychotic doses were used by many women in these studies.
Fetal and neonatal toxicity reported with exposure to the typical antipsychotics includes neuroleptic malignant syndrome (
185), dyskinesia (
186), extrapyramidal side effects manifested by heightened muscle tone and increased rooting and tendon reflexes persisting for several months (
187), neonatal jaundice (
188), and postnatal intestinal obstruction (
189).
Fetuses and infants also may be exposed to drugs used to manage the extrapyramidal side effects (eg, diphenhydramine, benztropine, and amantadine). In a case-control study, oral clefts were associated with a significantly higher rate of prenatal exposure to diphenhydramine than controls (
149). In contrast, in several other studies diphenhydramine use has not been found to be a significant risk factor for fetal malformations (
190,
191). Clinical studies of the teratogenic potential of benztropine and amantadine use are lacking.
In summary, typical antipsychotics have been widely used for more than 40 years, and the available data suggest the risks of use of these agents are minimal with respect to teratogenic or toxic effects on the fetus. In particular, use of piperazine phenothiazines (eg, trifluoperazine and perphenazine) may have especially limited teratogenic potential (
181). Doses of typical antipsychotics during the peripartum should be kept to a minimum to limit the necessity of utilizing medications to manage extrapyramidal side effects. There is likewise little evidence to suggest that the currently available atypical antipsychotics are associated with elevated risks for neonatal toxicity or somatic teratogenesis. No long-term neurobehavioral studies of exposed children have yet been conducted. Therefore, the routine use of atypical antipsychotics during pregnancy and lactation cannot be recommended. In a woman who is taking an atypical antipsychotic and inadvertently conceives, a comprehensive risk-benefit assessment may indicate that continuing therapy with the atypical antipsychotic (to which the fetus has already been exposed) during gestation is preferable to switching to therapy with a typical antipsychotic (to which the fetus has not yet been exposed).
▶ What is the risk of using psychiatric drugs while breastfeeding?
Breastfeeding has clear benefits for both mother and infant and, in making the decision to recommend breast-feeding, these benefits should be weighed against the risks to the neonate of medication exposure while breast-feeding (
Table 2). Most medications are transferred through breast milk, although most are found at very low levels and likely are not clinically relevant for the neonate. For women who breastfeed, measuring serum levels in the neonate is not recommended. Most clinical laboratory tests lack the sensitivity to detect and measure the low levels present. However, breastfeeding should be stopped immediately if a nursing infant develops abnormal symptoms most likely associated with exposure to the medication. Evaluation of the literature on drug levels in breast milk can facilitate the decision to breastfeed (
192).
In the treatment of depression, published reports regarding SSRI use and lactation now consist of 173 mother-infant nursing pairs with exposure to sertraline, fluoxetine, paroxetine, fluvoxamine, and citalopram (
193,
194–
215). In results from studies, it has been shown that, quantitatively, medication exposure during lactation is considerably lower than transplacental exposure to these same SSRIs during gestation (
193,
201,
208,
216). Generally, very low levels of SSRIs are detected in breast milk. Only a few isolated cases of adverse effects have been reported, although infant follow-up data are limited. The package insert for citalopram does report a case of an infant who experienced a transient apneic episode. Long-term neurobehavioral studies of infants exposed to SSRI antidepressants during lactation have not been conducted.
The TCAs also have been widely used during lactation. The only adverse event reported to date is respiratory depression in a nursing infant exposed to doxepin, which led to the conclusion that doxepin use should be avoided but that most TCAs are safe for use during breastfeeding (
217). Data regarding the use of atypical antidepressants during lactation are limited to the use of venlafaxine (
218) and bupropion (
219,
220).
The existing data regarding lithium use and lactation encompass 10 mother-infant nursing dyads (
103,
221–
225). Adverse events, including lethargy, hypotonia, hypothermia, cyanosis, and electrocardiogram changes, were reported in two of the children in these studies (
103,
223). The American Academy of Pediatrics consequently discourages the use of lithium during lactation (
226). Because dehydration can increase the vulnerability to lithium toxicity, the hydration status of nursing infants of mothers taking lithium should be carefully monitored (
102). There are no available reports regarding the long-term neurobehavioral sequelae of lithium exposure during lactation.
Only one adverse event, an infant with thrombocytopenia and anemia (
227), has been reported in studies regarding valproate use and lactation, which includes 41 mother-infant nursing dyads (
227–
235). Studies of the neurobehavioral impact of valproate exposure during lactation have not been conducted. The American Academy of Pediatrics and the World Health Organization (WHO) Working Group on Drugs and Human Lactation have concluded that use of valproate is compatible with breast-feeding (
226,
236). Reported adverse effects of carbamazepine in breast milk include transient cholestatic hepatitis (
237,
238) and hyperbilirubinemia (
239). The WHO Working Group on Drugs and Human Lactation has concluded that use of carbamazepine with breast-feeding is “probably safe” (
236).
In the management of anxiety disorders, benzodiazepine use exhibits lower milk/plasma ratios than other classes of psychotropics (
240,
241). Some investigators concluded that benzodiazepine use at relatively low doses does not present a contraindication to nursing (
242). However, infants with an impaired capacity to metabolize benzodiazepines may exhibit sedation and poor feeding even with low maternal doses (
243).
Of typical antipsychotic medications, chlorpromazine has been studied in seven breastfeeding infants, none of whom exhibited developmental deficits at 16-month and 5-year follow-up evaluations (
244). However, three breastfeeding infants in another study, whose mothers were prescribed both chlorpromazine and haloperidol, exhibited evidence of developmental delay at 12–18 months of age (
245).