Failure to remit, delayed remission, partial remission with residual symptoms, relapse, and recurrence are common outcomes of depression (
1). Cohort studies of depressed inpatients have shown that a substantial proportion, 12%–40% depending on the subjects and length of followup, never recover (
2–
4). Of those who do recover, 60%–80% subsequently relapse over the next 5–10 years (
3,
5). It is estimated that over the 10 years following a depressive episode, only 25% of patients do not have a recurrence (
6).
Despite this consistent evidence that depression is, for most patients, a chronic and/or recurrent illness, treatment trials are usually short-term. Two types of long-term studies have emerged: observation of a cohort of patients over a specific time and the follow-up of patients who, after successful treatment, are randomly assigned to continued treatment or a placebo.
The cohort studies are a variation on the naturalistic follow-up study, which consisted of mapping the course of a disorder in the absence of treatment. In depression, the availability and widespread use of treatments now make naturalistic study impossible. Instead, the course of illness may be altered by treatment choices that are uncontrolled and that develop and vary along with the illness (
7). Such studies include a wide range of patients with often complex psychopathology, and they survey treatments that clinicians actually use, including multiple medications and combinations of treatment. However, it is not possible to completely resolve the intervening role of treatment and the confounding of prognostic and treatment effects.
In contrast, randomly assigning recovered patients to placebo or continued treatment allows experimental control over confounders. The results have been consistent: continuing with an active treatment significantly reduces the odds of relapse, in relation to placebo. A meta-analysis of such studies indicated that continuing treatment with antidepressants reduces the odds of relapse by 70% (95% confidence interval=62%–78%) (
8). However, such designs impose limitations. They generally have rigorous inclusion criteria, reducing their generalizability, and they usually limit treatment to a single medication. Perhaps more important, they cannot examine the large group of patients who do not recover while taking the chosen drug, since those patients are unable to enter the long-term phase of the trial.
An alternative, but less commonly used, strategy is to follow a cohort of patients while attempting to control treatment. The advantages include less rigorous inclusion criteria, the use of a broader range of medications, and the opportunity to prospectively monitor the group that does not recover and to search for predictors of their outcome. A placebo treatment arm would be ideal but is difficult to justify in any study continuing beyond a few weeks.
We report a 5-year cohort follow-up study of a group of outpatients treated for major depression. The patients were initially randomly assigned openly to fluoxetine or nortriptyline, and they then continued with drug treatment on the basis of optimal clinical guidelines (
9,
10). This cohort design allowed retention of all patients, including those who did not recover, and description of the course and outcome of their illness.
This investigation had two aims. The first was to study the course of illness for patients treated in a systematic manner for 6 months. The second was to see whether there were clinically useful predictors of the course of illness over this time. In particular, we were interested in the effect of initial response on 6-month outcome. This is a variable that is believed to be strongly predictive of longer-term outcome.
METHOD
Subjects
Depressed patients were referred from a variety of sources, including a psychiatric emergency service, community mental health centers, and general practitioners. No patients were recruited by advertising. The subjects were screened over the telephone by a research nurse and then seen for an initial assessment by a psychiatrist or senior psychiatric registrar (R.T.M., P.R.J., S.E.L., or P.E.S.). Following this initial assessment, eligible patients were invited to participate in the study after giving written informed consent. The study was approved by the Ethics Committee of the Canterbury District Health Board.
The inclusion criteria included an age of 18 years or over and the ability and willingness to give informed consent. DSM-III-R major depression was the principal current diagnosis, and treatment with an antidepressant was appropriate management.
The exclusion criteria included current breast-feeding or pregnancy or the likelihood of pregnancy, a major medical disorder that could interfere with assessment and treatment, current severe alcohol or drug dependence (subjects with mild to moderate alcohol or drug dependence were included), and a history of schizophrenia, schizoaffective disorder, or mania (a history of hypomania was permitted).
The patients were free of all prescribed psychoactive drugs, except an occasional hypnotic for sleep, for a minimum of 2 weeks.
Baseline assessment
All patients were assessed by using the Structured Clinical Interview for DSM-III-R—Patient Version (SCID-P). The SCID-P had been extended to include all DSM-III-R and DSM-IV melancholic and atypical criteria for depression. The patients were rated on the Montgomery-Asberg Depression Rating Scale (MADRS) (
11), the 17-item Hamilton Depression Rating Scale (HAM-D), and the Clinical Global Impression (CGI). They also completed a series of self-report questionnaires, including the Hopkins Symptom Checklist (SCL-90) (
12), a personality disorders self-report questionnaire based on the Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II) (
13), and the Temperament and Character Inventory (
14).
Personality disorder was assessed according to the SCID-II from the information obtained with the personality disorders self-report questionnaire. The clinician (R.T.M., P.R.J., S.E.L., or P.E.S.) was required to rate personality on the basis of predepressive functioning or on functioning in the preceding 5 years. The kappa for test-retest reliability for the presence of a personality disorder was 0.70.
The patients were randomly allocated to receive fluoxetine or nortriptyline for an initial period of 6 weeks. At 6 weeks the mean fluoxetine dose was 28.1 mg/day (range=10–80). At 6 weeks the mean nortriptyline dose was 93.5 mg/day (range=50–175).
At 6 weeks patients continued taking their medication if they had responded. Unless clinically contraindicated, patients who did not respond were switched to the alternative medication. If the patient was unresponsive to the second drug, clinicians generally combined these two medications. If this failed, the clinician was free to use whatever medication was clinically indicated, usually switching to an alternative antidepressant or augmenting the current treatment with lithium. All patients who had responded were strongly encouraged to continue taking their medications for the 6 months. Formal assessments were performed at 3, 6, 9, 13, 20, and 26 weeks with the MADRS, HAM-D, and CGI.
6-Month assessment
At 6 months all patients were reinterviewed with the SCID-P. Outcome was classified as 1) depressed: patient meets DSM-III-R criteria for major depression, 2) residual symptoms: patient does not meet DSM-III-R criteria for major depression but has a MADRS score of 10 or more, or 3) well: patient does not have major depression and does not have significant residual symptoms (i.e., MADRS score ≤9).
Each patient's case was reviewed by two of us (R.T.M. and P.R.J.) to determine the course of illness over the 6 months. This was based on the summary CGI and MADRS scores. When there was a discrepancy, the individual case notes were reviewed and a consensus decision made. Remission was defined as a CGI score of 1 or 2 (i.e., much improved or very much improved) for 2–8 weeks, and recovery was defined as a CGI score of 1 or 2 for more than 8 weeks.
Principal outcomes
We divided the patients into three groups based on the course of illness: 1) good outcome: achieved recovery and remained well up to 6 months, 2) fluctuating outcome: achieved recovery or remission but suffered a relapse or recurrence within the 6 months, and 3) poor outcome: currently depressed and no remission or recovery over the 6 months.
Analyses
All data from the study were entered into the relational database Paradox (
15) and transferred to SPSS 10.0 (
16) for statistical analyses. The major outcome categories were based on the course of illness over the 6 months. All potential predictors of outcome were examined by using chi-square tests for category variables and analysis of variance for continuous variables. All significant predictors were entered into stepwise logistic regressions predicting good and poor outcomes. For these analyses two binary variables were created: 1) poor versus good and fluctuating and 2) good versus poor and fluctuating.
RESULTS
Subject characteristics
From the eligible patients, 195 were randomly assigned to treatment. Of these, 111 (57%) were female, and the mean age was 31.6 years (SD=11.4). According to DSM-IV criteria, 18 (9%) had bipolar II disorder, 86 (44%) had melancholia, 16 (8%) had atypical depression, 121 (62%) had recurrent depression, and 125 (64%) had chronic depression (defined as being depressed for more than 2 of the past 5 years). The mean baseline MADRS score was 31.0 (SD=6.6), and the mean HAM-D score was 19.9 (SD=4.4). Sixty percent of the subjects had never received a prescription for an antidepressant. For a detailed description of the original patient group, see our earlier article (
9).
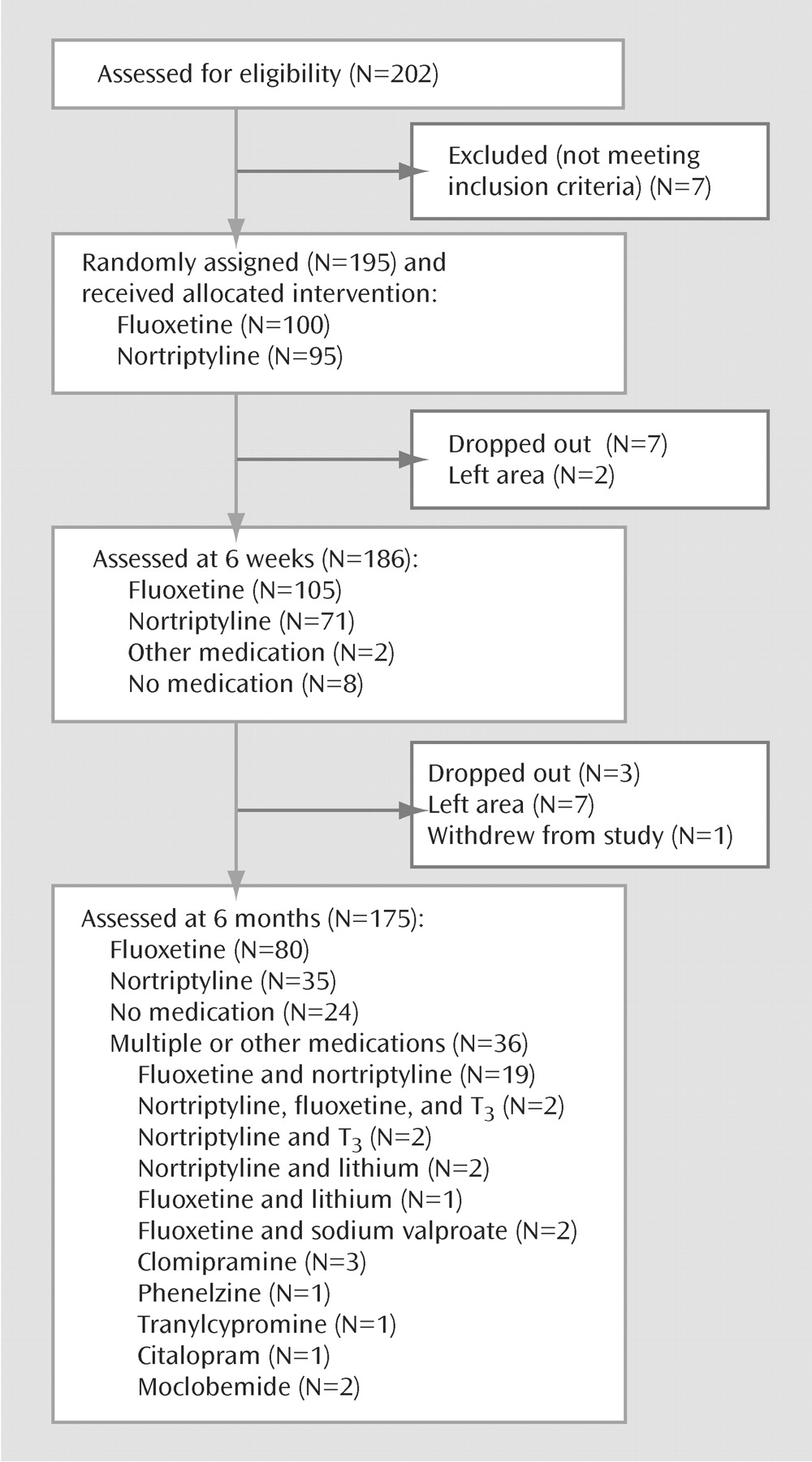
Treatment
Figure 1 shows how the subjects moved through the trial; 195 were randomly assigned to fluoxetine or nortriptyline, and at 6 months 175 remained in the study. A total of 10 dropped out (i.e., did not keep follow-up appointments), nine left the area, and one withdrew. By 6 months the majority of subjects (66%) were still taking, or had switched to, fluoxetine or nortriptyline alone. Twenty-four patients (14%) had elected to stop taking their medication or found it intolerable but remained in the study. Their outcome at 6 months was not significantly different from those taking medication (χ
2=0.008, df=2, p=0.56 for medication versus no medication in relation to the 6-month course of illness). The remaining 36 (21%) were either taking a combination of fluoxetine and nortriptyline or were taking other medications.
Outcome of illness
Table 1 shows the outcome at 6 months and the course of the illness. It can be seen that the majority of patients were well, with nearly all of these achieving recovery and remaining well. Forty patients (23%) were depressed at 6 months, according to DSM-III-R criteria, but half of these had had a remission or recovery over the 6-month period. A further 22 patients had residual symptoms at 6 months. Most of these had had a remission or recovery. Thus, 72% (126 of 175) of the patients had achieved a recovery by 6 months (i.e., had at least 8 weeks with clinician ratings of “much improved” or “very much improved”). Of these 126, 20% (N=25) had not sustained this recovery. A further 20 had a remission but no recovery, four had persistent residual symptoms, while 20 had suffered persistent major depression.
The primary outcome was the course of illness over 6 months.
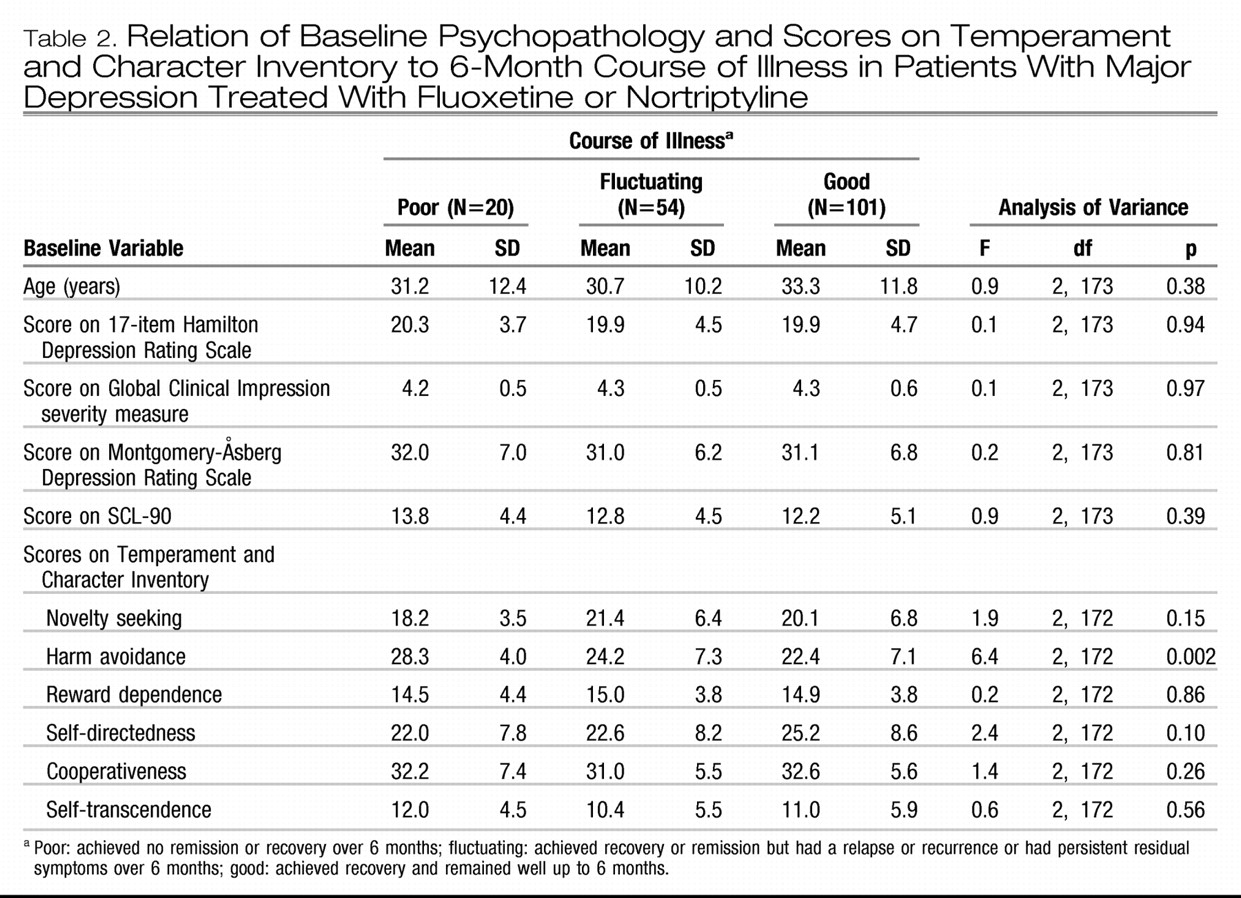
Table 1 shows that 58% of the patients had a good outcome, 31% had a fluctuating course, and 11% had a poor course of illness. Baseline depression severity, measured by using HAM-D, MADRS, and CGI scores, was not related to outcome, nor was total psychopathology, measured with the SCL-90 self-report (
Table 2). The only significant relationship between the scores on the Temperament and Character Inventory and 6-month outcome was the finding that patients with a poor outcome had higher harm avoidance scores at baseline (
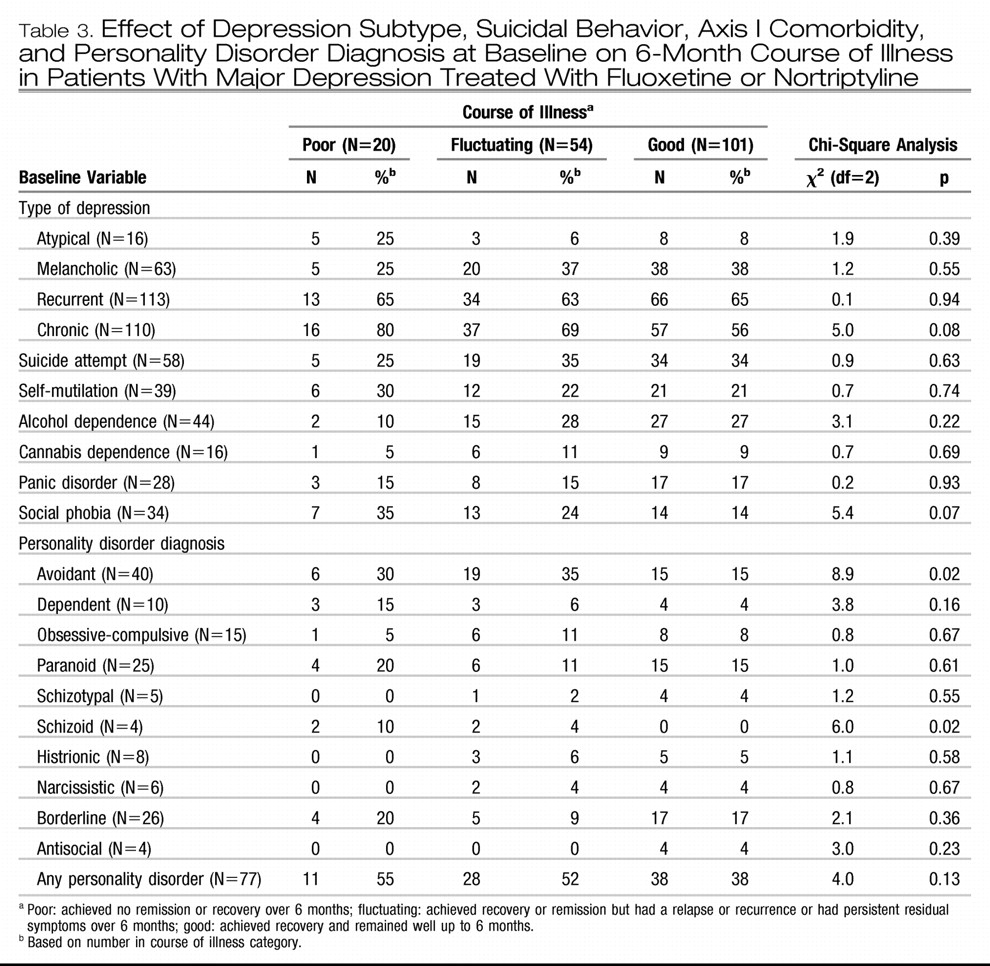
Table 2). Baseline depression subtype, suicidal behavior, and axis I comorbidity, including recurrent depression and chronic depression, also were not significantly associated with 6-month course of illness. There was a nearly significant relationship between comorbid social phobia and worse outcome (
Table 3).
The associations between personality disorder diagnoses at baseline and 6-month outcome are also shown in
Table 3. A poor outcome was associated with avoidant and schizoid personality disorder. When the numbers of personality disorder symptoms were examined, a poorer outcome was associated with the presence of more symptoms of avoidant personality disorder (F=3.8, df=2, 172, p=0.03), schizotypal personality disorder (F=4.2, df=2,172, p=0.02), and schizoid personality disorder (F=3.5, df=2, 172, p=0.04). Total personality disorder symptoms were not related to outcome.
Effect of 6-week response on 6-month course of illness
Our primary measure of 6-week response was a 60% reduction in MADRS score. Of the 175 patients, 91 were categorized as having an early response. This was significantly related to 6-month outcome: 63% of those with good outcomes, 48% of those with fluctuating courses, and 5% of those with poor outcomes had early responses (χ2=23.2, df=2, p<0.001).
Effect of all significant variables on 6-month course of illness
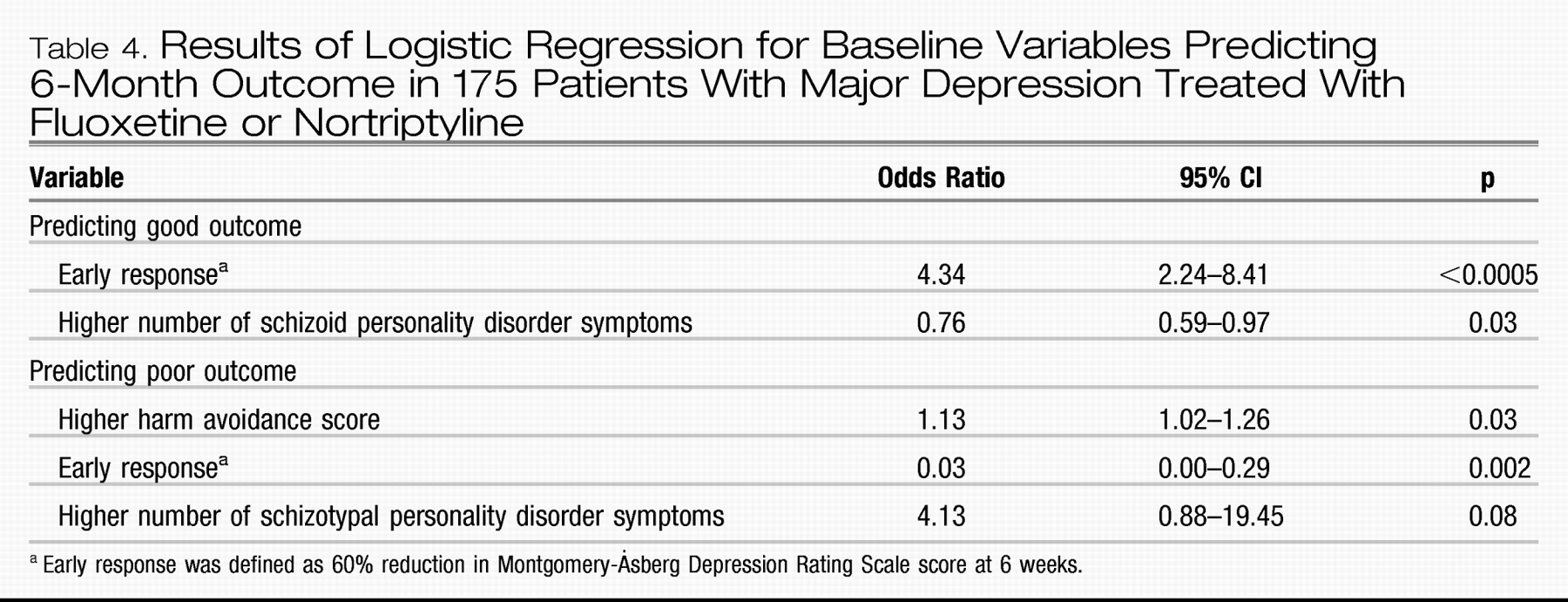
We then entered all significant baseline variables, i.e., harm avoidance score, avoidant personality disorder diagnosis and number of symptoms, schizoid personality disorder diagnosis and number of symptoms, and number of schizotypal personality disorder symptoms, as well as the 6-week response into stepwise logistic regressions predicting good and poor outcomes. No additional baseline variables showed a significant association with outcome when the course of illness was dichotomized as good versus fluctuating and poor or as poor versus fluctuating and good.
Table 4 shows the result of the forward stepwise logistic regression in predicting good and poor outcome. For good outcome, the variables entered into the regression were early response and the level of schizoid symptoms. For poor outcome, the variables selected were early response, harm avoidance score, and schizotypal personality disorder diagnosis, although the last variable was no longer significant. Both models adequately predicted outcome. Predicting good/not good correctly classified 68% of the subjects, while the poor/not poor model correctly classified 90%.
DISCUSSION
Outcome of depression
We found three primary ways that patients treated for depression progress over 6 months. First, there is a large group, comprising 58% of our study group, who recover and remain well. Second, there is a minority (11% of our subjects) who neither recover nor achieve remission over this period despite ongoing active treatment. Finally, nearly one-third of patients achieve remission or recovery but then relapse, have a recurrence, or suffer from residual symptoms.
We found five reports of comparable studies of progress by 6 months (
3,
17–
20). All included the overall rate of recovery, regardless of whether or not the patients had relapsed. According to this perspective, 72% of our subjects had recovered by 6 months, 11% had continuous symptoms, and 17% had residual symptoms or had a remission but no recovery. If we exclude one study (
17) that did not have a clear definition of “recovered”and adjust the data of Ramana et al. (
18) to include only recovered patients who had no significant residual symptoms, the recovery rate at 6 months in the earlier studies varies from 38% to 54%. Our cohort had a better outcome. The reasons for this may include the definition of recovery, the type of patients treated, or the treatments given.
Our definition of recovery is similar to that used in other studies, i.e., more than 8 weeks with minimal depressive symptoms. However, our subjects were outpatients with moderate to moderately severe depression. Three of the four comparable studies included a substantial proportion of inpatients, which suggests that those study groups might have comprised patients with more severe or treatment-resistant illness. In addition, our treatments were controlled within the research team; all patients were monitored and encouraged to continue taking medication and were actively followed up if they failed to keep appointments. Two of the comparable studies simply monitored normal clinical care, while the other two had treatment protocols. It is possible that the close monitoring of treatment in our study may have improved outcome.
Predictors of response
Our second main finding was that baseline variables, including demographic factors, the historical and phenomenological features of the depression, and the patients' comorbid disorders, were not generally associated with the course of illness over 6 months. Avoidant, schizoid, and schizotypal personality features, as well as high harm avoidance scores, were modestly associated with a worse course of illness.
The most powerful predictor of the course of illness was the 6-week response; this gave an odds ratio of 4.34 (95% confidence interval=2.24–8.41) for good versus not good outcome. Our regression model classified 68% of the subjects correctly. This seems clinically useful but must be judged against the rate of good outcome. Most patients do well, so a clinician predicting this for all patients has a 58% chance of predicting correctly. The model is even less useful in predicting poor outcome. Although 90% of the subjects were placed in the correct category by the regression model, so few did poorly that someone predicting that all subjects will not do poorly classifies 88% correctly. Of the 20 subjects who did poorly, only two (i.e., 10%) were correctly classified by the logistic regression, again no better than a clinician guessing.
Therefore, although we have a number of clinical factors that are related to outcome and the regression model we used enables us to correctly classify the outcome of most of our patients, it has limited clinical utility. This is because most patients will do well, or at least not do poorly. Any clinician predicting this for all patients will do nearly as well as our model. In attempting to predict the relatively small group of patients who do poorly, our model was of no assistance.
Few studies have produced comparable data. Ramana et al. (
18) reported that more severe chronic depression at baseline predicted a longer time to remission but that personality pathology, diagnostic subtype, life events, social supports, and marital relationships had no effect. Ezquiaga et al. (
19) reported that comorbid personality disorders, previous episodes, and some aspects of social support were associated with not achieving full remission but that severity, melancholia, and self-esteem were not. Predictors that explain a small amount of variance and are inconsistently replicated have plagued this area of research, but we hoped that by measuring 6-month outcome, more consistent and powerful predictors, particularly early response, might emerge. Instead, we found that any model we developed was little better than a clinician simply predicting that all patients would do well over the 6 months of treatment.
Limitations
The most obvious limitation of any study discussing treatment outcome is the absence of a placebo control group. However, following depressed patients for 6 months while withholding treatment is not feasible when effective treatment is available. A second limitation is that treatment choices develop and vary along with the course of depression. Again, this is unavoidable, and the study can make no claims about the efficacy of the treatment given or the relative efficacy of various antidepressants. A third limitation is that the outcome described may apply only to moderately depressed outpatients, but these are the patients many psychiatrists commonly treat. Finally, the outcomes were chosen because we wanted to capture the course of illness rather than a cross-sectional snapshot, which may have produced different results.
CONCLUSIONS
This study shows that over 6 months of treatment most patients with major depression do quite well. Over one half became well and remained well. A further one-third had a fluctuating course but were reasonably well much of the time, while approximately one in nine of the patients remained unwell despite active treatment. This study also shows that we have limited ability to predict which patients will fall into each group.
Acknowledgments
Funded by a grant from the Health Research Council of New Zealand, a grant from Lottery Health, and an unrestricted grant from Eli Lilly (New Zealand). The Clinical Research Unit of the Department of Psychological Medicine, Christchurch School of Medicine and Health Sciences, is supported by the University of Otago and the Mental Health Division of Canterbury Health.
The authors thank Robyn Abbott and Isobel Stevens and the local psychiatrists and registrars who treated these patients.