Obsessive-compulsive disorder (OCD) is an anxiety disorder characterized by repetitive thoughts, images, impulses, or behaviors that cause significant time loss, distress, and/or impairment of functioning. This illness is highly morbid and disabling in its severe forms and affects children, adolescents, and adults. Genetic studies have demonstrated that both biological and environmental factors are important in the etiology of OCD (
1). With respect to environmental factors, stressful events have been associated with OCD onset in childhood (
2), perinatal, and adult forms of the illness. With respect to nongenetic biological factors, several illnesses have also been associated with OCD, including temporal lobe epilepsy (
3), autoimmune diseases such as systemic lupus erythematosus (
4,
5) and Crohn's disease (
6,
7) and infectious processes (
8–
10). With respect to potential genetic factors, family studies of OCD report increased prevalence of this disorder among relatives, and twin studies have confirmed that a genetic component underlies the illness. Segregation analyses performed on multigenerational families suggest that this disorder is difficult to model using Mendelian approaches. In molecular genetic work, linkage studies have attempted to identify regions in which OCD vulnerability genes may lie and numerous candidate genes studies have been studied for potential association with the OCD phenotype. Most recently, a collaborative group of more than 20 OCD research sites has undertaken a genome-wide association study (GWAS) in search of common or rare susceptibility genetic variants.
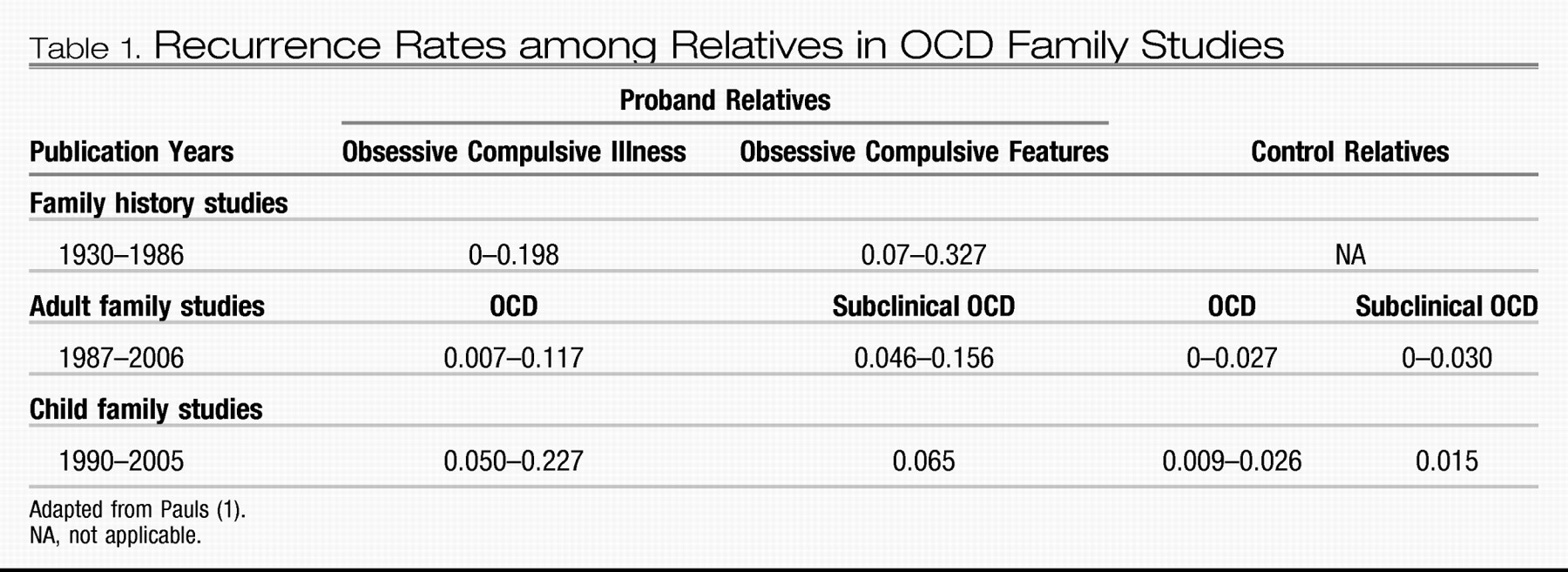
Family studies of OCD-affected individuals published since the 1930s provide strong evidence for familiality of the disorder (
Table 1). As OCD symptoms are frequently hidden, even from relatives, family studies in which relatives are not directly interviewed about their own diagnoses probably underestimate recurrence rates. To date there have been 15 OCD family studies in which relatives received structured interviews (
11–
26). Seven of these focused on childhood OCD (
11–
13,
19,
21,
22,
25), all of which reported significantly higher OCD rates among relatives than general population or control rates. Recurrence risks were also much higher than familial rates reported in adult OCD studies. Although reported rates of OCD among relatives of adults with OCD were approximately 2 times that among control subjects, the rate of OCD among relatives of children and adolescents with OCD was increased approximately 10-fold in those studies for which control rates were available. The most recent controlled family study (
26) ascertained OCD probands from both European population and clinic samples, reporting increased OCD rates among relatives from both samples. This study's findings also parallel results of earlier studies completed in the United States (
17,
18,
23) with families ascertained through treatment facilities. Finally, a meta-analysis of data from 1,209 first-degree relatives (
27) studied before 2001 reported a significantly increased OCD recurrence risk among proband versus control relatives [Mantel-Haenszel summary odds ratio=4.0 (95% confidence interval=2.2–7.1)]. The unadjusted aggregate risk for relatives of OCD probands was 8.2%, compared with 2.0% for relatives of control subjects. These results demonstrate that at least some cases of OCD are familial, which may be attributed to combined genetic and/or environmental effects.
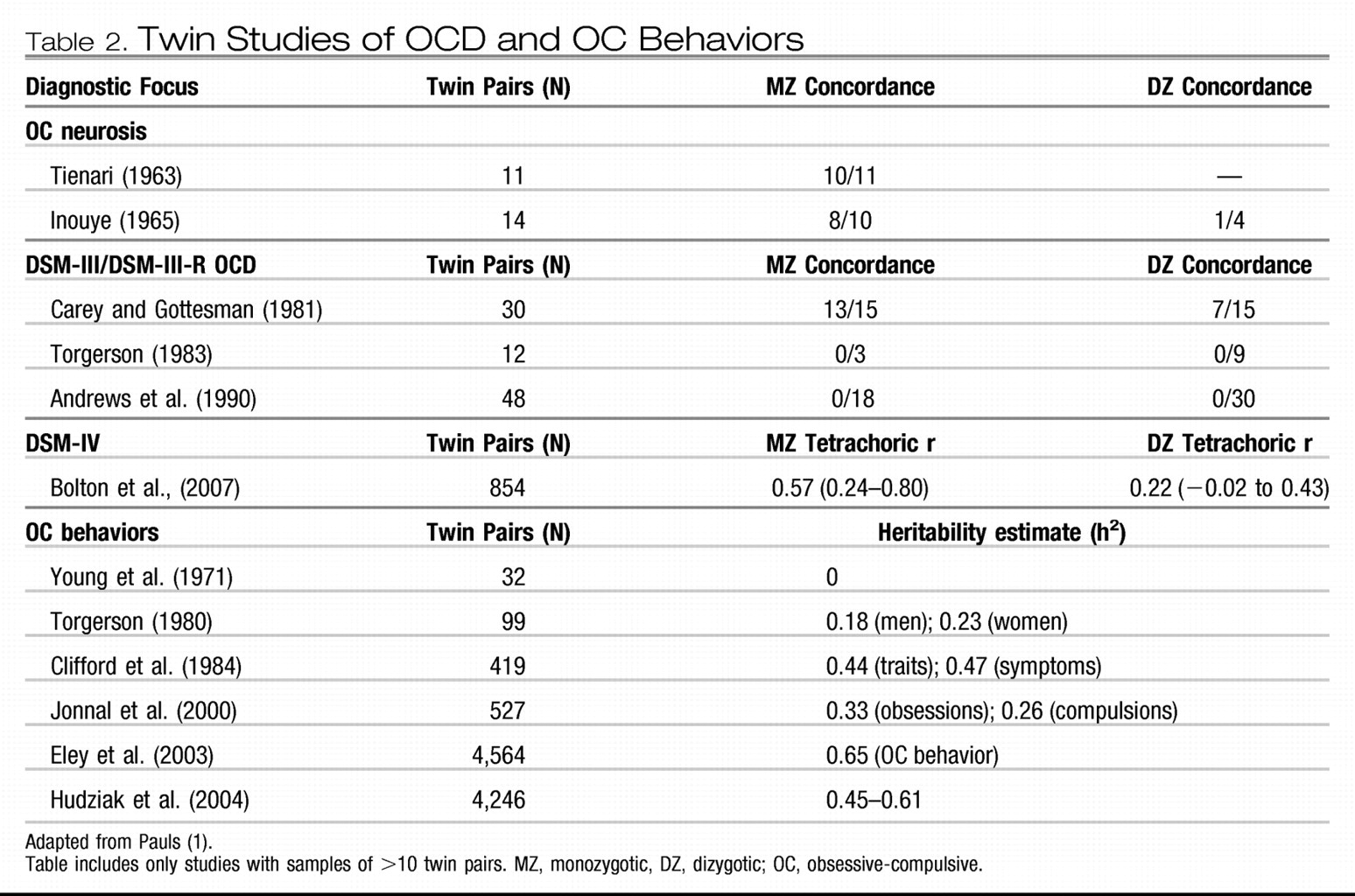
Twin studies help to determine whether genetic factors are important in the etiology of complex disorders, thus providing greater etiological specificity than family studies (
Table 2). The concordance rate differences between monozygotic and dizygotic twins provide an estimate of heritability (defined as the percentage of phenotypic variance attributed to genetic factors). In a meta-analysis of twin studies published between 1929 and 2005, van Grootheest et al. (
28) reported heritability estimates ranging between 45 and 65% in children and between 27 and 47% in adults. In the only additional twin study published since 2005, Bolton et al. (
29) examined a community sample of 854 6-year-old twins and concluded that shared etiological factors exist for OCD and tics and for OCD and other anxiety disorders. Four complex segregation analyses of OCD have been conducted to estimate the “goodness of fit” for specific familial patterns of transmission (
30–
33). These suggested that some genes of major effect are probably involved in this disorder, although OCD transmission is difficult to model.
Genome-wide linkage studies are molecular genetic studies that attempt to identify regions in the genome that may contain vulnerability genes. None of the three of these studies completed to date in OCD (
34–
36) yielded genome-wide significant findings, although they did identify genomic regions of interest for future research. Hanna et al. (
34) completed a genome scan on seven families identified through childhood OCD probands. They reported a lod score (logarithm of the odds ratio for linkage) of 2.25 (1.97 after fine mapping) for marker D9S288 on chromosome 9p. Willour et al. (
37) genotyped 50 OCD pedigrees (
37) in an attempt to replicate these findings. The largest lod scores observed in this study were for markers D9S1792 (heterogeneity lod=2.26) and D9S1813 (nonparametric linkage= 2.52, p=0.006), lying within 350 kb of the marker with the strongest finding reported by Hanna et al.
The second OCD genome-wide linkage study included sib-pairs and multigenerational members of 219 families. These authors reported the strongest linkage in examination of compulsive hoarding only on chromosome 3q27–28 [Kong and Cox lod(all) score [KAC(all)] score=2.67] for OCD (
35) and on chromosome 14 [KAC(all)=2.9 for families with one affected relative and 3.7 for families with two or more affected relatives] (
38). In the third OCD genome-wide linkage study 26 multigenerational families were genotyped. The maximum nonparametric lod (nlod) score was equal to 2.43 for markers located on chromosome 10p15. Unfortunately, when combined with data from the previous genome scan (
34), the maximum nlod score was decreased to 1.79. In further follow-up via family-based association analysis of single nucleotide polymorphisms (SNPs) in this 10p15 region, however, association was detected with three adjacent SNPs. This included the amino acid variant rs2271275 in the 3′ region of adenosine deaminase acting on RNA 3 (ADAR3) (p<0.05).
Because sample sizes in all three linkage studies were quite small, these findings should be interpreted with caution. Nonetheless, the fact that Willour et al. (
37) observed suggestive linkage in the same chromosome 9p region as reported by Hanna et al. (
34,
36) is noteworthy. In addition, as discussed in the following paragraphs, four independent studies have reported an association between OCD and the glutamate transporter gene
SLC1A1, which is located in this region of 9p. However, illustrating the inconsistency of OCD genetic findings (potentially related to OCD heterogeneity across samples), this region did not show any evidence for linkage in the study completed by Shugart et al. (
35).
As noted above, family and twin studies have provided evidence that OCD is both familial and genetic. Segregation analyses have clarified that transmission does not occur in a simple Mendelian fashion and are in keeping with patterns seen for complex genetic disorders. In an effort to identify regions containing OCD vulnerability loci, whole genome linkage scans have reported some regions of suggestive linkage, and analyzing the subphenotype of hoarding led to an improved linkage signal. However, at best linkage scans are only able to identify broadly defined genomic regions, which contain numerous genes. An alternate approach, via candidate gene studies, searches for associations between OCD and specific genes, selected based upon their location or function.
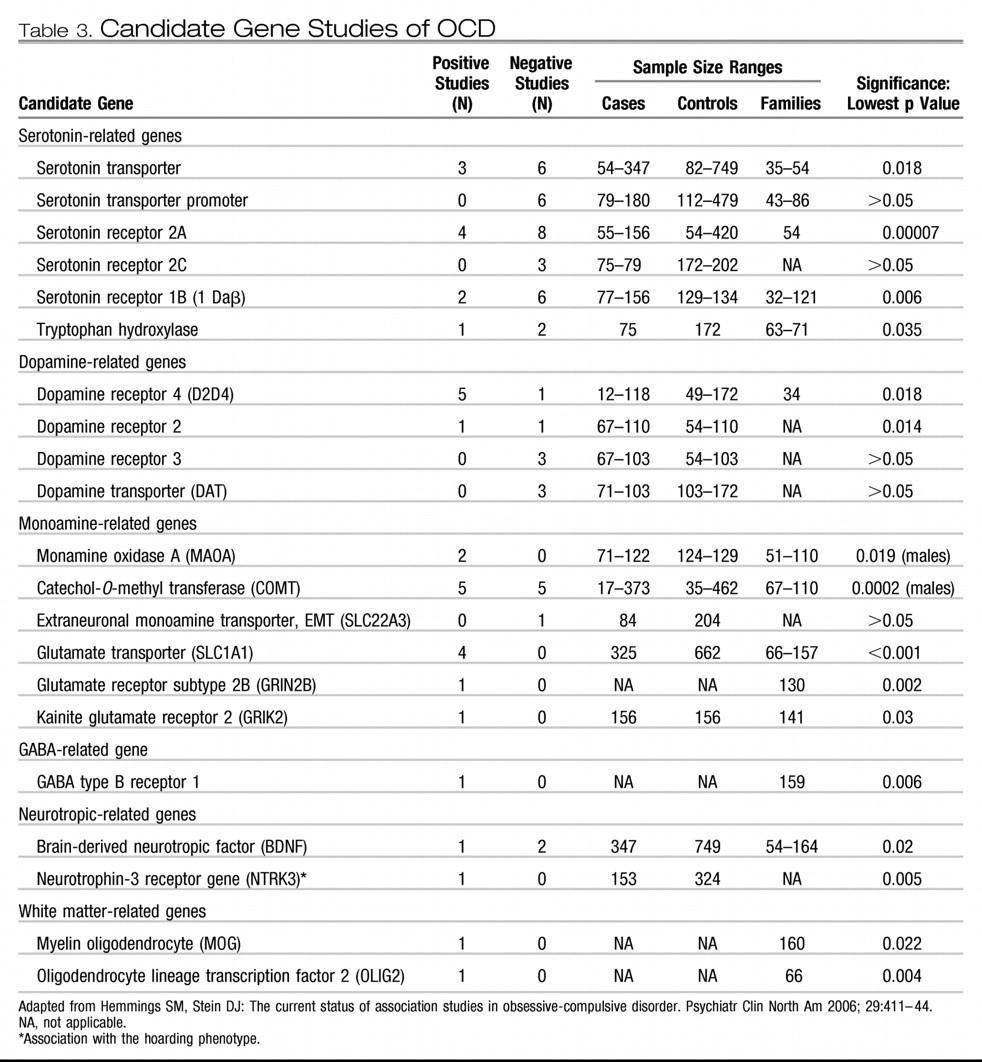
More than 80 OCD candidate gene studies have been reported over the last decade (
1). These have mainly been selected for study because of their proximal location to linkage peaks (positional candidates) or their putative etiological role in neurotransmitter pathways or anatomy related to OCD (functional candidates). Specifically, genes within serotonin, dopamine, and glutamate pathways and those involved in brain white matter have been the center of focus, outlined in the following paragraphs.
White matter volume abnormalities have been noted in both child (
39) and adult (
40) OCD samples. In addition, diffusion tensor imaging studies have reported white matter connectivity abnormalities in OCD (
41). Following from this, white matter genes including
OLIG2 (
42) and
MOG (
43) have been studied with reported association in small family-based samples.
Until recently, serotonin remained the leading target for investigations of the neurochemical underpinnings of OCD, largely because of the efficacy of selective serotonin reuptake inhibitors (SSRIs) in its treatment (
44). As such, numerous serotonin-related genes have been studied in OCD (
Table 3). The most commonly studied of these is the serotonin transporter gene. In a meta-analysis stratifying OCD samples, a significant association was identified with the l-allele of the serotonin transporter polymorphism in family-based association studies with child and Caucasian samples (
45).
The dopamine neurotransmitter has also been implicated in OCD etiology. OCD-like behaviors in humans have been reported to emerge after administration of dopamine receptor 2 agonists and stimulants (
46). Augmentation efficacy has also been reported for atypical antipsychotics, which act on serotonin and dopamine receptors (
47). However, association studies of dopamine-related genes to date have reported only mixed results. There is also a complex interaction between glutamatergic, dopaminergic, and serotoninergic neurons (
48,
49).
There has been a convergence of evidence across various lines of research supporting the putative role of glutamate in OCD (
50,
51). Functional OCD neuroimaging studies have consistently reported metabolic hyperactivity in the cortico-striato-thalamo-cortical circuitry (
52–
55). Concentrations of glutamate have been found to be increased in the CSF of OCD-affected adults (
56) and decreased in the anterior cingulate cortex of children with OCD (
57). Rosenberg et al. (
57,
58) conducted an elegant series of studies suggesting a notable role of glutamate in childhood OCD. Altered glutamate receptor levels have also been reported in OCD-implicated brain areas within subjects with OCD (
59). Moreover, promising augmentation trials of glutamate-modulating agents riluzole (
60) and memantine (
61) have been completed. OCD animal model studies also support a role for glutamate in OCD. Knockout mice for the striatum-expressed
SAPAP3 gene (coding for a protein at corticostriatal glutamatergic excitatory synapses) reportedly developed facial lesions, repetitive grooming behaviors, and anxiety that were reversed separately with SSRI administration and with gene replacement (
62). In a recently published Nature paper (
63), Slitrk5 deficient mice also demonstrated OCD-like behaviors diminishing with SSRI treatment and showed glutamate receptor composition changes. Moreover, glutamate receptor gene (
GRIK2)-deficient mice have shown significantly less fear memory and fewer anxious behaviors than wild type mice (
46,
64). Lastly, treatment with glutamate-related medications has reduced marble-burying behavior (another OCD animal model) in mice (
65). Glutamate-related genes that have been associated with OCD in small studies to date include the glutamate transporter
SLC1A1 (
66–
69) and glutamate receptors
GRIN2B (
70) and
GRIK2 (
71). In addition, an association between the previously mentioned
SAPAP3 gene and pathological grooming behaviors (but not OCD) in humans has been found (
72).
Unfortunately, there has been a failure to consistently replicate nearly all OCD candidate gene study findings. Further, none of the OCD candidate gene association studies reported to date have had sufficient power to demonstrate genome-wide significance. Among OCD candidate gene studies, the glutamate transporter gene
SLCL1A1 (
66–
69) is the only one of these that has been repeatedly associated across OCD samples. Of note, this gene has also been associated with atypical antipsychotic-induced obsessive-compulsive symptoms (
73) and with autism, a disorder frequently associated with obsessive-compulsive behaviors (
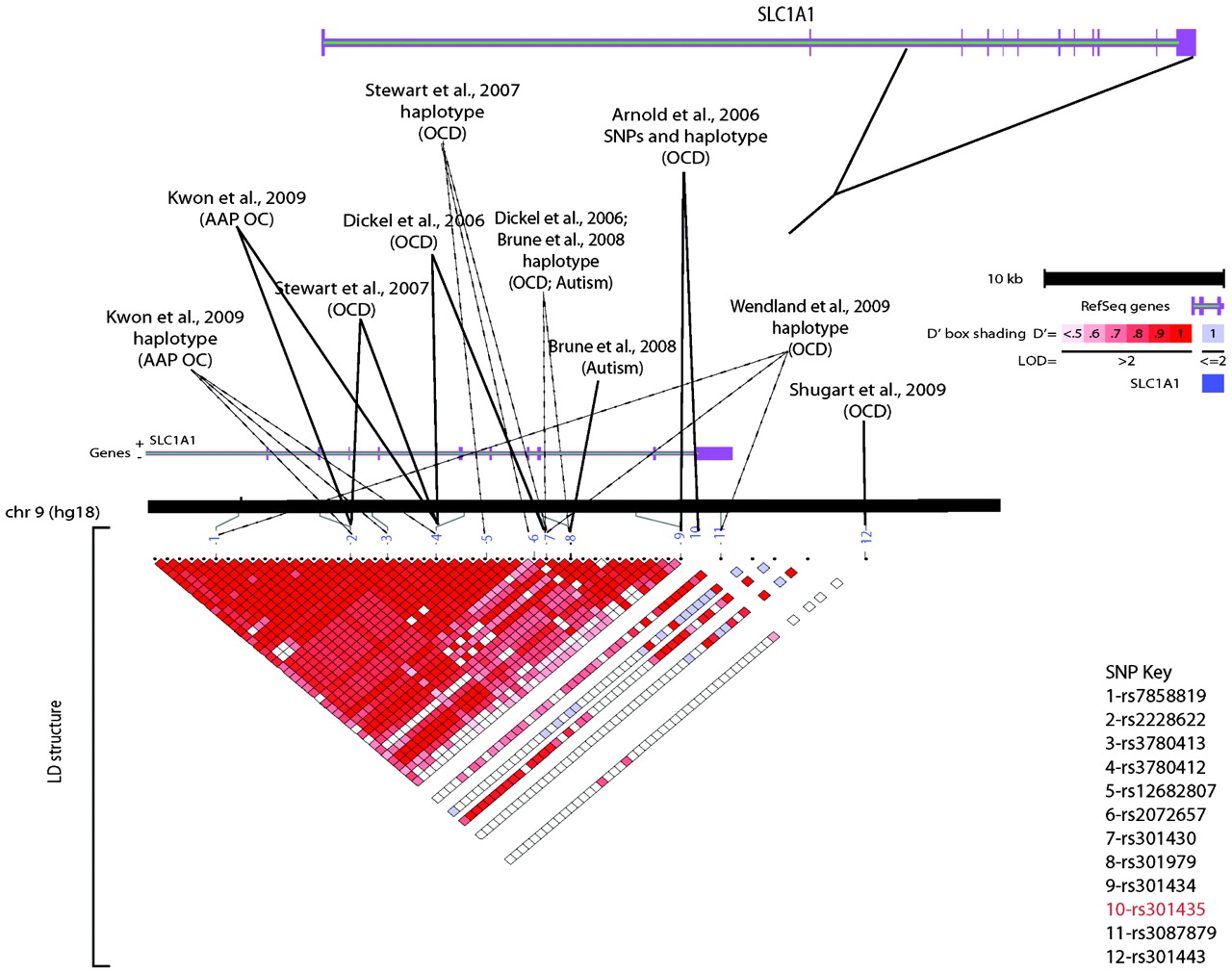
Figure 1) (
74).
In summary, OCD is a complex genetic disorder that seems to also be strongly influenced by environmental and nongenetic biological processes. Moreover, it is possible that subtypes of this heterogeneous disorder exist, with independent or overlapping etiological factors. The twin and family studies summarized in this review suggest that combined influences from OCD vulnerability genes play a notable role in OCD etiology. However, given that the linkage studies and essentially all of the candidate genes studies to date provide only suggestive evidence for OCD risk genes of moderate to large effect, GWASs of OCD are warranted as the next step in understanding the genetic basis of OCD. GWASs are preferred over more traditional linkage studies or candidate gene studies as they provide enhanced ability to identify risk genes of relatively small effect. These GWASs have the capability to examine both common SNP markers as well as copy number variants and other rare genetic events. There is emerging evidence that complex disorders may result from both rare genes of major effect and a combination of common genes of lesser effect, requiring very large sample sizes. In an effort to meet this need, the International OCD Foundation Genetics Collaborative is currently conducting a GWAS of OCD on samples contributed from 21 different research sites from around the world. It is anticipated that individual or combined results from GWAS markers will eventually further illuminate knowledge about the impact of underlying genetic etiological factors on OCD, allowing for clinical translational applications of these findings.
Acknowledgments
We thank Dianne Hezel for her invaluable help in the preparation of this article.