This noninvasive ability to stimulate the brain makes TMS a powerful research tool in studying a host of cognitive processes such as the motor system,
5–8 vision,
9 language,
10 and even memory.
11 There has been much interest in whether TMS might work as an antidepressant (see reviews
4,12,13). Building on open studies where TMS was applied over the vertex to treat depression (with inconclusive results),
14–16 George and Wassermann
17 proposed in 1994 that TMS applied to the prefrontal cortex might be more effective. They based their argument on evidence that electroconvulsive therapy (ECT) response is linked to changes in prefrontal function,
18 and on functional imaging studies in depression where prefrontal cortex has been shown to be dysregulated (see reviews
19,20). Recently, several parallel design double-blind treatment trials have suggested that prefrontal TMS applied daily over 2 to 3 weeks can work as an antidepressant.
21–24The mechanisms of action of prefrontal TMS as an antidepressant are unknown. Previous work in healthy adult volunteers has shown that prefrontal TMS (and not TMS at other brain regions) increases serum thyroid measures, hinting that changes in mood might be due to neuroendocrine changes.
25 In addition, two imaging studies during TMS in healthy control subjects have begun to shed some light on what may be happening in the brain during stimulation. Kimbrell et al.
26 used fluorodeoxyglucose (FDG) positron emission tomography (PET) to image the effects of 20 minutes of prefrontal TMS at 1 Hz and found that stimulation, compared with a sham condition, was associated with a global reduction in activity. In addition, TMS caused relative decreases in activity both below the site of stimulation and in deeper regions including the caudate, orbitofrontal cortex, and cerebellum. George and co-workers
27,28 used perfusion SPECT to image cerebral blood flow during fast (20 Hz) left dorsolateral prefrontal cortex (DLPFC) TMS in healthy adults. Compared with a sham condition, TMS was associated with relative decreases in activity in the anterior cingulate, right prefrontal, and orbitofrontal cortex and relative increases in activity in the brainstem and cerebellum. In summary, these two imaging studies of prefrontal cortex TMS in healthy adults suggest that TMS is likely having both local cortical effects immediately below the site of stimulation and secondary limbic changes.
To further study the effects of TMS on mood and the brain, we imaged resting brain activity in depressed patients before and after participation in a randomized double-blind placebo-controlled treatment trial. On the basis of previous studies of the putative regional neuroanatomy of mood dysregulation in depression
20 and previous work in healthy control subjects with left DLPFC SPECT,
27 we posed the following pre-study hypotheses:
METHODS
Subjects
Twenty-seven depressed subjects who were enrolled in a 2-week double-blind placebo-controlled trial of TMS were scanned (as described below) immediately before and then 3 days after 2 weeks of TMS treatment. Five subjects were excluded from final analysis because they lacked either the baseline or end SPECT scan or the data were not usable. Thus, 22 patients (9 men) who met DSM-IV criteria for either major unipolar depression (
n=14; 5 men) or bipolar depression, depressive phase (
n=8; 4 men) were used for the final analysis. Although failure to respond to other antidepressant medications was not an explicit entry criterion, this cohort was largely treatment refractory and had been ill for many months before enrolling in this trial. The average number of years since the first diagnosis of depression was 21.9 years (SD=11.8,
n=16), and the average duration of the current episode was 21.7 months (SD=22.1,
n=18). Of the 22 subjects, 13 received active stimulation and 9 received placebo. Complete information about this clinical treatment trial is reported elsewhere (Nahas et al.
29 and manuscript under review).
Subjects were free of antidepressant medications for at least 2 weeks prior to study entry, although 3 bipolar patients required ongoing mood stabilizers or benzodiazepines for anxiety (1 each received valproic acid, clonazepam, and lithium plus alprazolam), and 1 patient required medication for thyroid disease (thyroxine). All subjects gave written informed consent following full explanation of the procedures and risks. See
Table 1 for complete subject information.
Ratings and Response Classification
Before entering the study, subjects were screened and diagnosed by using the Schedule for Affective Disorders and Schizophrenia (SADS).
30 In addition, the 21-item Hamilton Rating Scale for Depression (Ham-D)
31 was obtained at baseline and at end of study. Trained psychiatric nurses, blind to treatment arm, performed all ratings.
Ham-D scores were used to calculate percentage improvement between the beginning and the end of treatment. Following convention,
32,33 subjects who showed 50% improvement or better at 2 weeks from baseline were classified as responders. Six of the 13 subjects who received active treatment met this pre-study criterion for treatment response. Six subjects who received active treatment but who were not responders were then chosen to best match the responders on key variables (age, gender). No subjects receiving placebo met response criteria.
Transcranial Magnetic Stimulation
TMS was performed with a Cadwell Magnetic Stimulator equipped with a figure 8–shaped coil and a continuous water cooling system to prevent overheating. Subjects received treatment for 10 days (all weekdays over 2 weeks) for 20 minutes per day at 100% of motor threshold. Subjects were randomly assigned to receive stimulation at either 20 Hz (2 s on, 28 s off), 5 Hz (8 s on, 22 s off), or placebo (coil angled at 45 degrees with one wing touching so that the bulk of the magnetic field did not pass through the skull). Because of the small sample sizes, for the purposes of this imaging analysis subjects receiving 20 Hz and 5 Hz stimulation were pooled into one “active” group.
Motor threshold was determined by placing the coil over primary motor cortex and determining the minimum amount of stimulation required to initiate visible motor movement at rest of the contralateral (right) abductor pollicis brevis (APB) muscle. The left prefrontal cortex stimulation site was defined as the location 5 cm rostral to and in a parasagittal plane from the site of APB stimulation.
Single-Photon Emission Computed Tomography (SPECT) Imaging
Whole-brain resting SPECT imaging was performed 3 days prior to starting TMS and 3 to 4 days after the last TMS session (but prior to restarting any medications). Intravenous access was obtained, followed by a 15-minute rest period during which subjects sat in a dark, quiet room with eyes closed. Thirty mCi (1,110 MBq) of technetium-99 bicisate (ECD; Neurolite®, DuPont Pharma) were injected, followed by an additional 15 minutes of rest before scan acquisition.
SPECT images were acquired by using a triple-headed Picker camera with low-energy ultra-high resolution fan beam collimators. They were processed on an Odyssey VP computer using a low-pass filter with the default order of 2+0.32 as the cutoff. Images were attenuation-corrected and reconstructed transversely and then transferred to a Sun SPARC20 for analysis. Statistical Parametric Mapping (SPM96b) software was used to apply a 10-mm smoothing followed by linear normalization into Talairach space.
34 These normalized images of relative brain perfusion were used as the dependent variable in the analyses.
Analyses
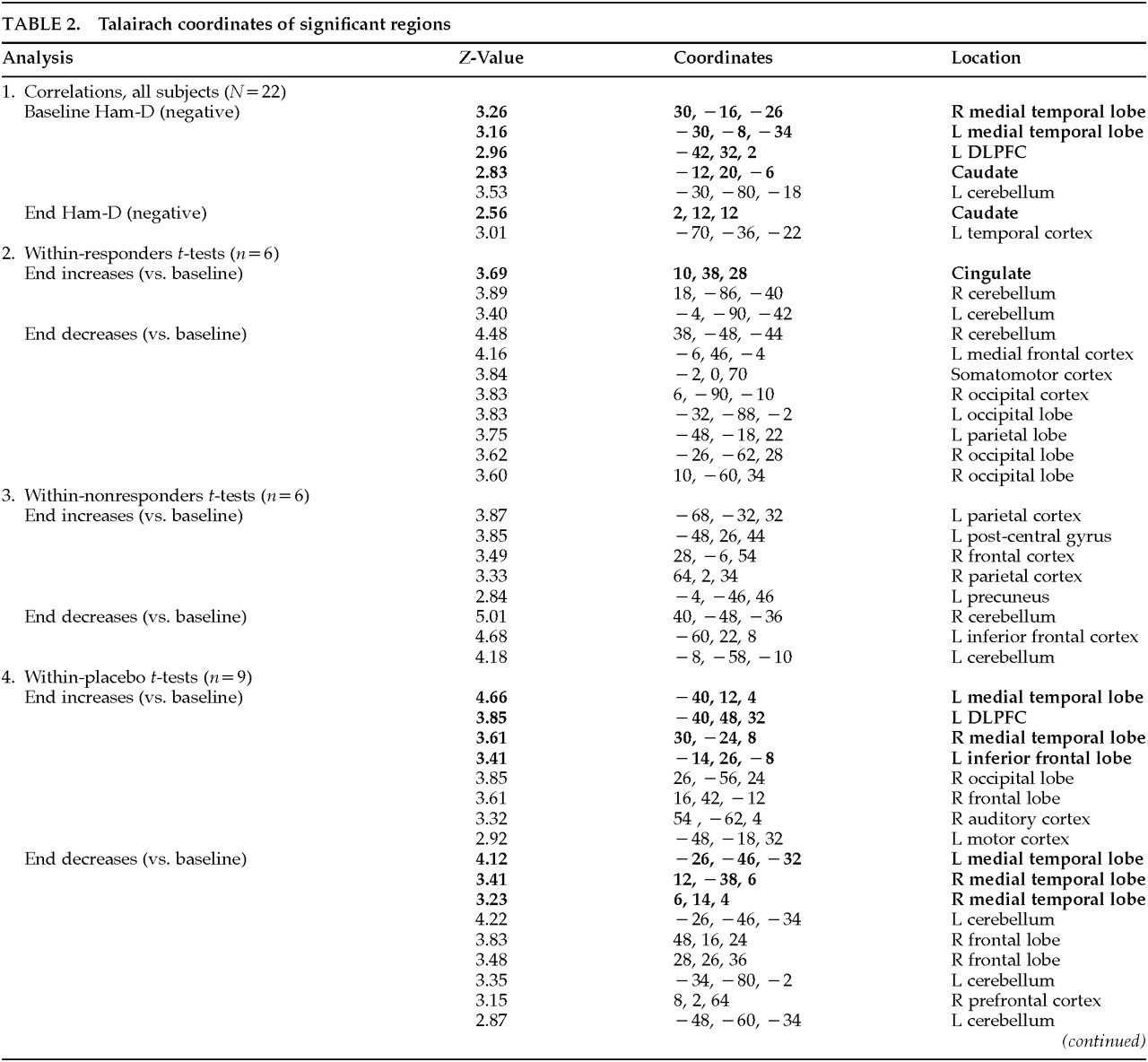
The data analysis used a two-stage approach. Both approaches used Statistical Parametric Mapping (SPM96) software, which does not distinguish between hypothesis-driven and more exploratory analyses. The data were compared across conditions by using a threshold of P<0.01, with a gray matter threshold of 0.6 and proportional scaling of the grand mean at 50. The following analyses were performed to test hypotheses regarding specific regions:
•
1. To test the hypothesis that baseline activity in the DLPFC and specified limbic regions correlates with depression severity, baseline Ham-D scores were compared with baseline regional blood flow by using Pearson's correlations.
•
2. To test the hypothesis that baseline relationships change over 2 weeks of TMS treatment, correlations between end Ham-D scores and end blood flow in these predetermined regions were computed. In addition, change in activity over time (baseline versus end) was analyzed within the responder and placebo groups by using two-tailed paired Student's t-tests.
•
3. To test the hypothesis that baseline activity in the predetermined regions might distinguish TMS responders from nonresponders, blood flow in these regions at baseline was compared across groups. These analyses were repeated at the end to see whether TMS had affected the differences in activity between the two groups.
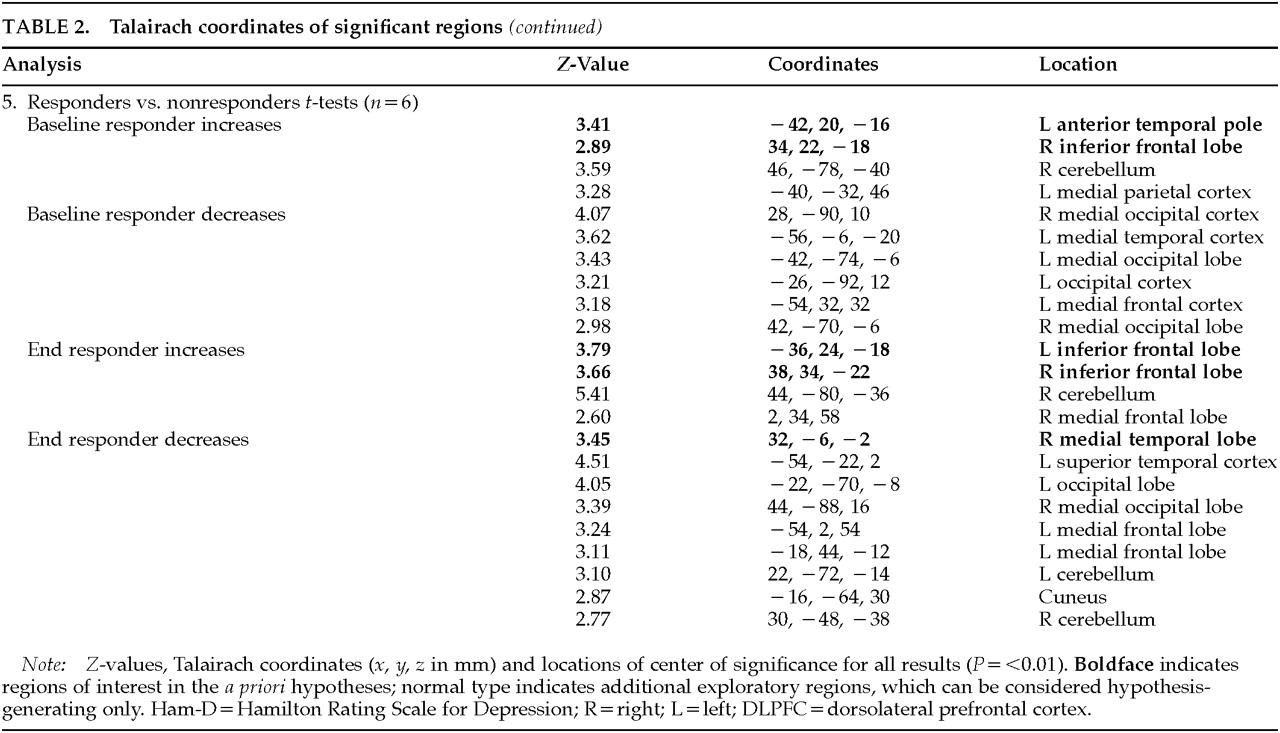
This was the full extent of the hypothesis-driven analyses. These analyzed regions are listed in bold in
Table 2A and
Table 2B. Because SPM performs analyses on all regions irrespective of
a priori hypotheses, we report changes in other regions (shown in the table in medium type) as well for all contrasts performed. Because these were not hypothesis-driven, they must be considered exploratory and await further testing in later studies.
DISCUSSION
This is the first study examining regional brain activity before and after TMS used as a potential antidepressant treatment. As such, it has several limitations that are discussed in detail below. However, this study had four key findings, some of which require replication before firm acceptance:
•
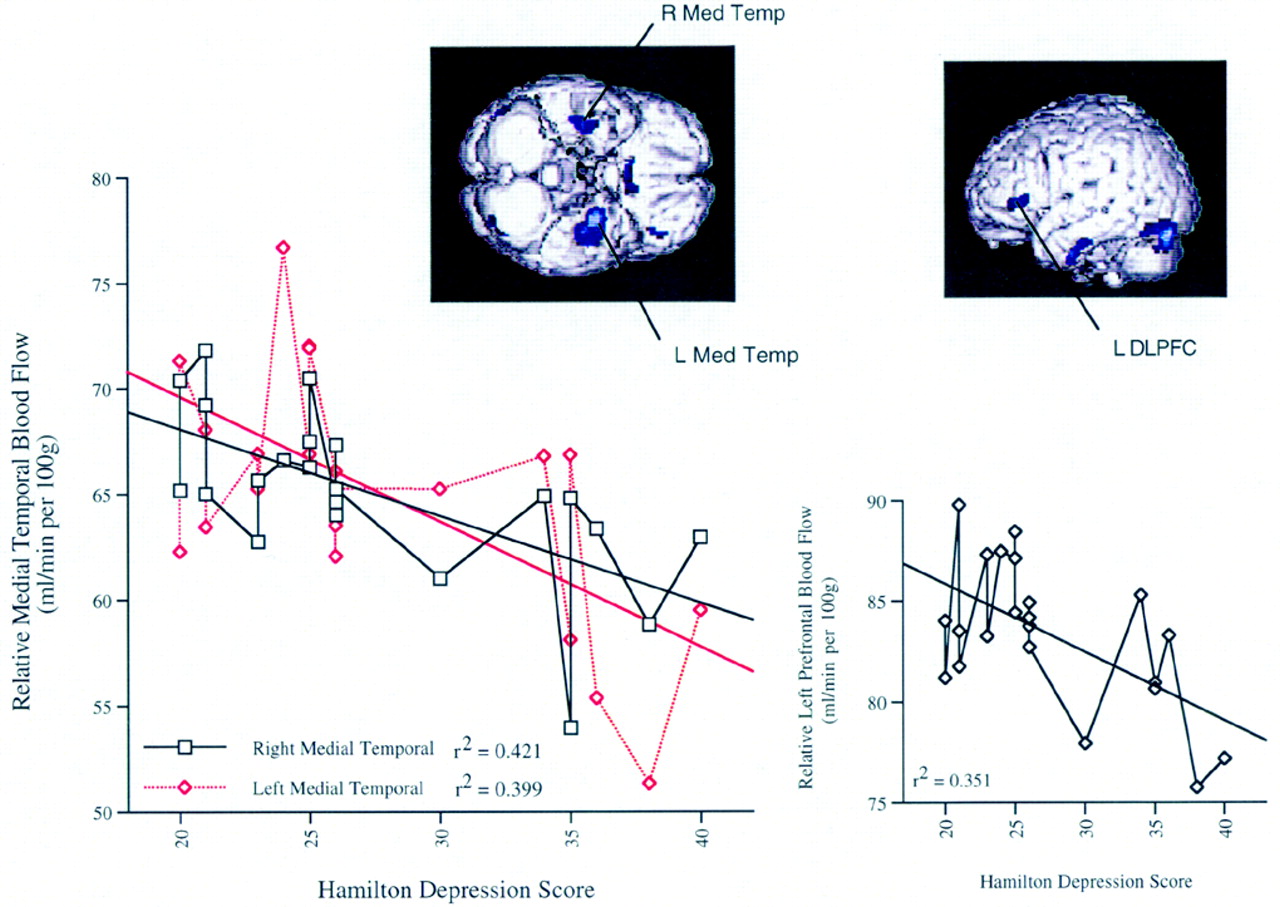
1. Confirming several other studies, baseline Ham-D inversely correlated with prefrontal and limbic activity.
•
2. These Ham-D correlations were not seen across the group as a whole immediately following treatment.
•
3. Regional cerebral blood flow changed in limbic regions as a function of mood improvement, both with TMS and with placebo.
•
4. TMS antidepressant responders differed from nonresponders in inferior frontal activity, at baseline and even more following treatment.
This study in a largely treatment-refractory outpatient sample confirms previous studies that have implicated DLPFC and the limbic system in mood dysregulation.
20,35 Several previous studies have found that as depression severity worsened, there was less activity in the prefrontal lobes and caudate.
36–38 Here, the left DLPFC region that negatively correlated with severity of depression was not the site of direct TMS stimulation but was approximately 4 cm inferior. The fact that these negative correlations (except caudate) were no longer significant after treatment across all subjects might suggest that 2 weeks of left DLPFC TMS may alter brain activity in these regions and the relationship to mood. However, as these findings are correlational in nature, they should not be viewed as causal and must await replication in noncorrelational analyses.
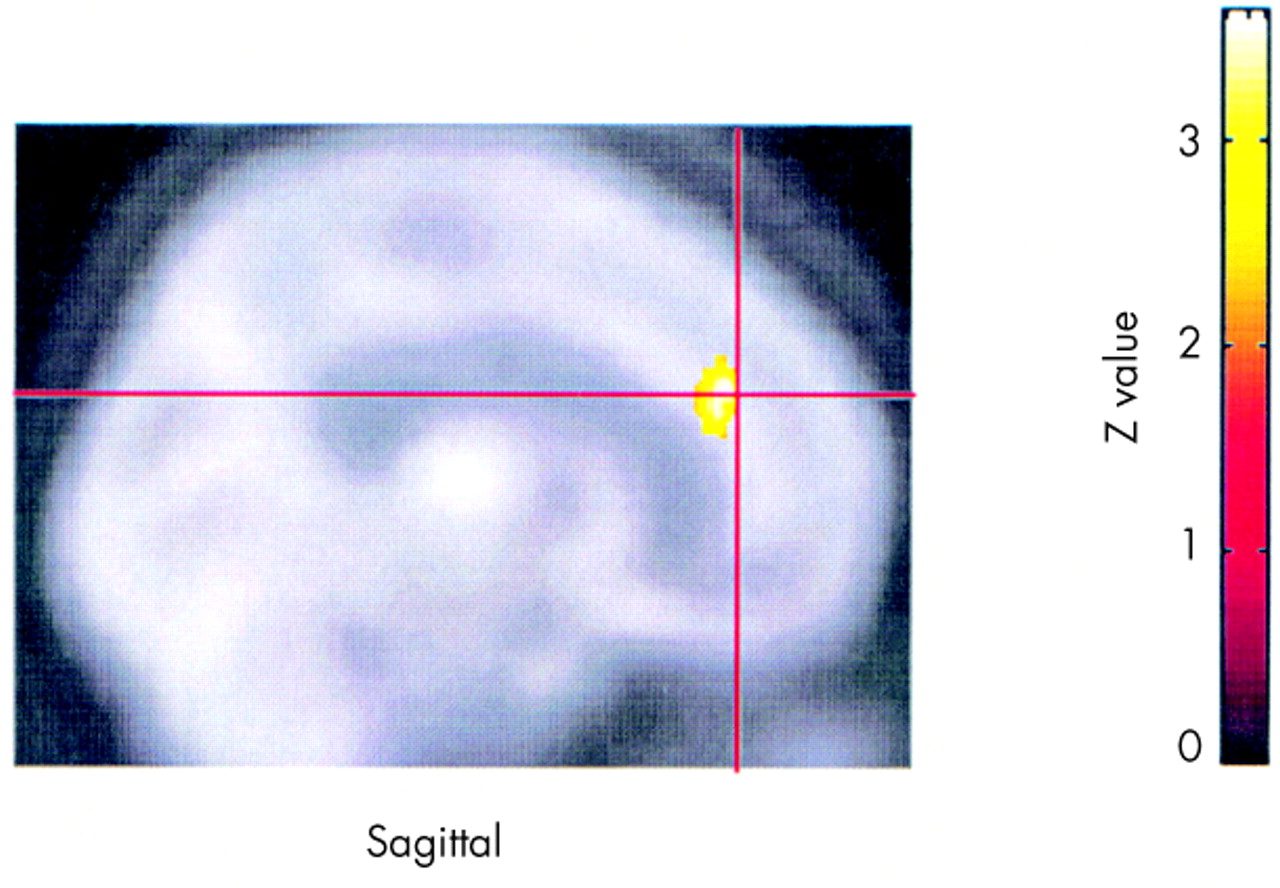
The finding that responders showed increased activity in the cingulate at end compared with baseline also supports the hypothesis of changes in paralimbic activity in association with improvement of mood in general. The cingulate is an important structure mediating both attention
39–41 and other higher behavior.
42–46 It has been shown to be blunted in depressed subjects undergoing a neuropsychological challenge.
47 Further, increased cingulate activity has been shown to predict antidepressant response to sleep deprivation
48,49 or fluoxetine
50 and to predict who among a group of remitted depressed subjects will relapse with a pharmacological challenge.
51Interestingly, we also found significant changes in rCBF in important limbic and prefrontal regions in the group receiving placebo. The placebo group did have a small improvement in mood (Ham-D scores decreased by an average of 20.5%; range 7.7–45), and these regional brain activity changes most likely reflect a state change away from a more severe depression. These findings in the placebo group show that one should use caution when attributing changes specifically to TMS rather than to state changes associated with depression. It is also possible that the placebo treatment actually directly affects the brain. Lisanby and Sackeim
12,52 have shown in primates with temporal lobe depth electrodes that prefrontal TMS, even with an angled coil as used in our placebo arm, can cause immediate changes in EEG signal from those deep regions.
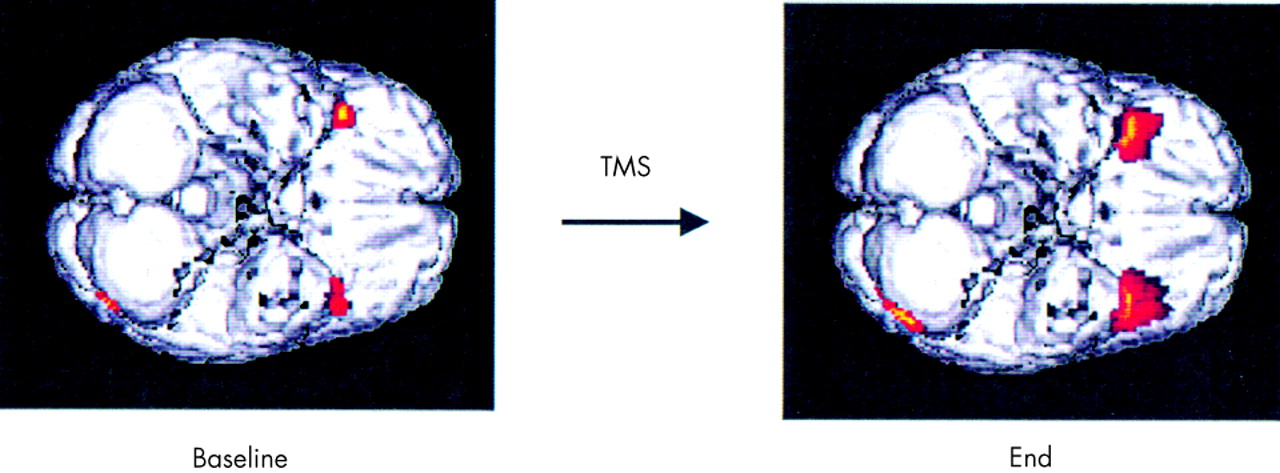
Responders compared with nonresponders had increased inferior frontal activity at baseline, suggesting that it may be possible at baseline to identify potential TMS responders. However, the small number of subjects in this analysis argues for caution and the need for replication before acceptance. The differences between responders and nonresponders increased in the same regions at the end, suggesting that perhaps responders had undergone a further normalization of activity in these regions. Responders also had decreased medial temporal activity following treatment.
Although the finding of decreased medial temporal activity in responders following treatment seems paradoxical, it is consistent with the pre-study hypothesis that prefrontal TMS produces changes in paralimbic regions and that these changes are linked in a complex way to the antidepressant effects. Studies in animals have recently shown that the prefrontal cortex has a negative, inhibitory effect on limbic structures (particularly the amygdala).
53 Thus, repeated prefrontal TMS that boosts prefrontal cortex activity might cause secondary reciprocal inhibition over time in limbic projections. Differential effects of prefrontal cortex TMS locally compared with limbic regions may also explain the lack of correlation of depression severity with paralimbic regions at the end of treatment.
This study has several limitations that bear on proper interpretation of the findings as a whole. These images provide only a snapshot (before and after TMS treatment over 2 weeks) of activity of processes that are dynamic in nature, most likely as part of a larger system. A further complicating matter is that even small structures in the limbic system are composed of multiple smaller nuclei, many of which act discretely, sometimes antagonistically. A SPECT camera with a 7-mm initial resolution rising to 20 mm after smoothing and transformation into Talairach space must sum activity over these discrete areas.
Additional limitations include the relative rather than absolute nature of the data that SPECT imaging provides. Moreover, the study lacks a control group and suffers from small sample sizes, especially in analysis of differences between responders and nonresponders. Because of the small sample size, subjects were pooled across the two active treatment arms with different frequencies (5 Hz and 20 Hz) and could not be matched on some factors known to affect blood flow (including age, gender, and depression type). Although it is unlikely that these differences could account for changes within the same person over time, they pose a potential confound for between-group comparisons. The post hoc examinations, which are also limited by small sample sizes, provide some soft evidence that, within this particular sample, these factors did not contribute significantly to the findings.
Lastly, the possibility that the changes and deficits in activity originated in structural differences cannot be ruled out, although it is unlikely that such changes would occur over a 2-week period. Volumetric structural MRI scans were acquired on all subjects, at baseline and end of study. Measurements of prefrontal cortical volume before and after 2 weeks of TMS did not show any significant differences.
54In spite of these limitations, this study provides an important first look at the potential antidepressant mechanisms of TMS. It supports the previously hypothesized involvement of left DLPFC in depression. In addition, the more profound changes appear to take place in deeper regions, implying that TMS acts secondarily on these areas, perhaps through hypothesized prefrontal cortex governance of limbic structures. These findings suggest that the antidepressant mechanisms of TMS may differ from those of ECT, which appears to cause a reduction in prefrontal activity that is associated with treatment response.
18,55,56 TMS, in contrast, appears to increase relative activity, especially in the cingulate, in responders only. Using various forms of functional imaging to investigate the regional brain effects of TMS appears to have potential for understanding the pathogenesis and regional neurobiology of pathological mood regulation.
6,56–61