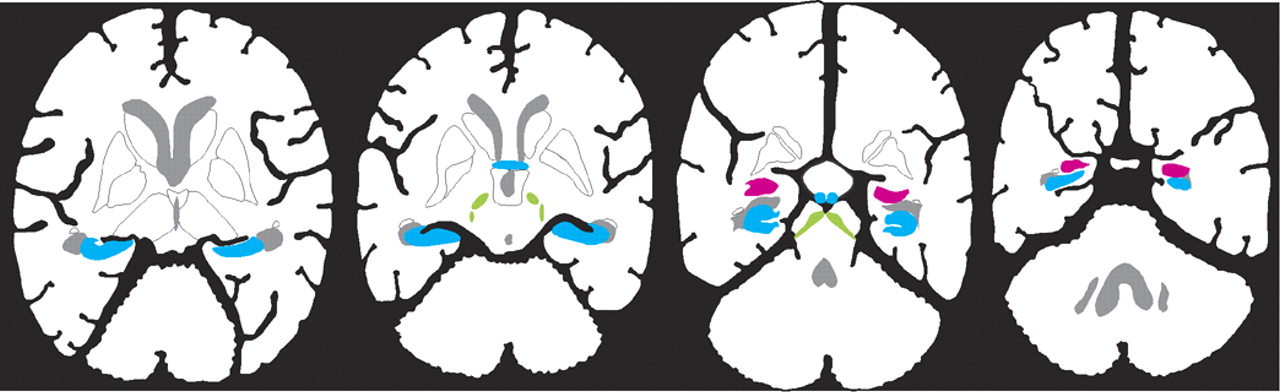
In addition to the well-known localization of estrogen receptors within hypothalamic nuclei, which are important for regulation of sexual and reproductive behaviors (not illustrated), estrogen receptors have now been found in other areas of the brain (see Cover and
Figure 1). There are at least two forms of the estrogen receptor (alpha and beta), and their distributions within the brain are different.
11–13 Receptor mapping studies do not all agree, perhaps because several different techniques (autoradiography, in situ hybridization, immunocytochemistry) and multiple species (mouse, rat, guinea pig, monkey, human) have been used. There are clear species differences, so the following summary is based only on studies of human and nonhuman primates (see Cover and
Figure 1). Several studies have found estrogen receptors in the hippocampal formation (hippocampus proper, dentate gyrus, subiculum, and entorhinal cortex), basal forebrain (septal nucleus, diagonal band of Broca, and nucleus basalis of Meynert), and mamillary bodies (Cover and
Figure 1, blue areas).
11,13–19 These support an influence on declarative (autobiographical, explicit) memory. Presence in the amygdala and dorsal raphe nucleus may underlie some of the effects of estrogen on mood and emotion (Cover and
Figure 1, pink area).
11,14–19 Estrogen receptors are present in movement-related areas including the substantia nigra and the subthalamic nucleus (Cover and
Figure 1, green areas).
13,17–19 They may also be present in the cerebellum (not illustrated), but exact localization is still unclear.
11,13 Most studies have not found estrogen receptors in the basal ganglia (caudate, putamen, or globus pallidus).
11,13–16,18,19 Several studies have reported estrogen receptors in various areas of the cerebral cortex (not illustrated), but this is still controversial.
11,13,18 No obvious differences have been reported between females and males in regional distribution of the alpha estrogen receptor.
15,16 There may be gender differences in the distribution of the beta form of the receptor.
11 One study has reported similar distributions of estrogen receptors in intact and ovariectomized females.
15Estrogen has multiple modes of action within the central nervous system.
12,20–24 It binds to a nuclear receptor, acting intracellularly to alter gene expression. Estrogen actions via this genomic mechanism, which are necessarily slow, may include inhibition of apoptosis, suppression of inflammatory reactions, and modulation of neurotrophins and growth factors as well as neuronal structure and synapse formation. Estrogen also has rapid actions, occurring far too quickly for genomic mechanisms. These may include its antioxidant effects as well as enhancement of cerebral blood flow and cerebral glucose utilization. These nongenomic effects probably occur via both plasma membrane receptors and non–receptor-mediated pathways. Some actions, such as modulation of neurotransmitters, may occur by both genomic and nongenomic mechanisms.
Estrogen interacts with multiple neurotransmitter systems at multiple sites. For instance, it has been shown to modulate the levels of dopamine (upregulation), serotonin (downregulation), norepinephrine (downregulation), and acetylcholine (upregulation) in prefrontal cortex.
25 This may occur via direct actions within cortex, or indirectly via estrogen receptor–mediated changes in brainstem or basal forebrain areas. Estrogen co-localizes with some neurotransmitters. Estrogen receptors have been found in serotonergic neurons within the dorsal raphe nucleus, where estrogen appears to facilitate serotonergic transmission by several mechanisms.
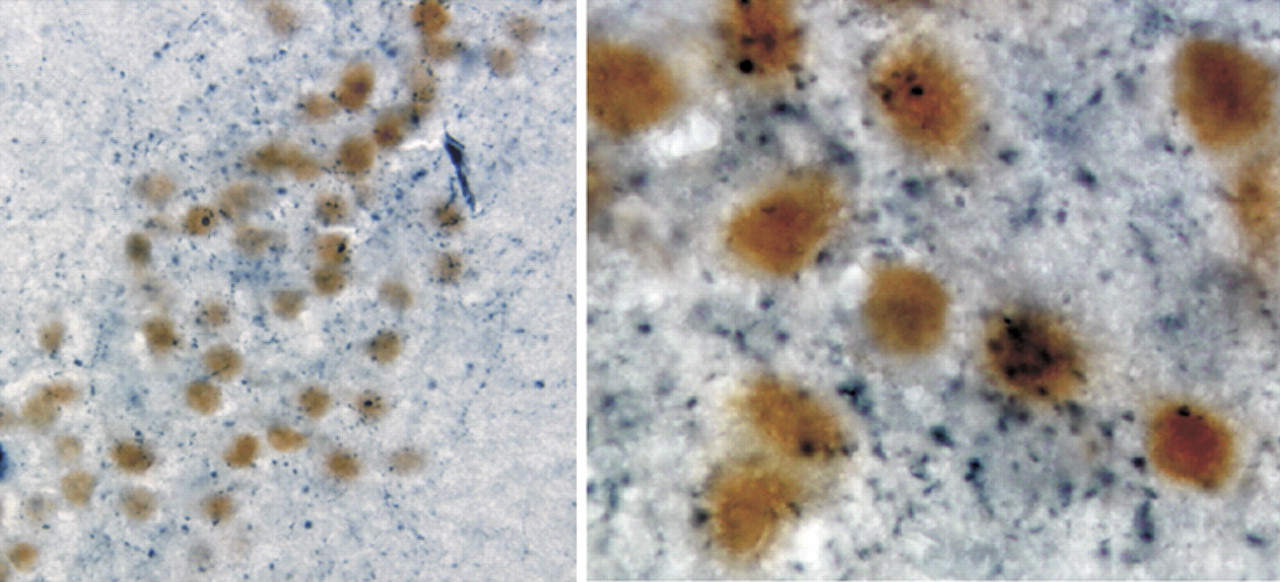
26 Estrogen alpha receptor–containing neurons in the amygdala are heavily invested with cholinergic terminals projecting from the basal forebrain (
Figure 2).
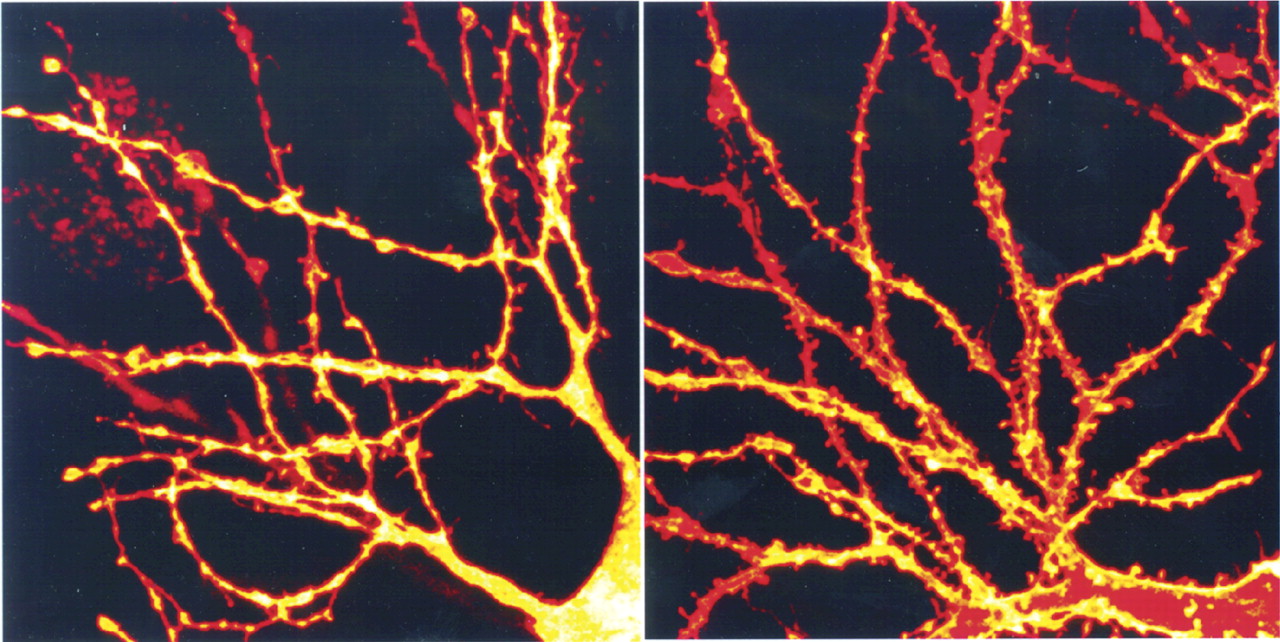
15 Estrogen modulates aspects of neuronal plasticity, including dendritic spine formation. In the hippocampus, for instance, estrogen receptors are localized in GABAergic interneurons. Estradiol exposure decreases the activity of these inhibitory interneurons (via an interaction with a neurotrophin), resulting in an increase in pyramidal cell excitability, which in turn promotes formation of new dendritic spines and synapses (
Figure 3).
27–29