Chronic pain is one of the most common and challenging problems facing our society. It is the second leading cause of work loss in the United States and is the third greatest health care problem after cardiac disease and cancer. It has widespread impact on patients, their families, friends, and the work place. Its influence extends to businesses, industry, and health care systems throughout the United States. Approximately 30% of the U.S. population (75 million Americans) suffer from chronic pain, with approximately $65 billion spent annually to treat and manage this problem.
1 Pain management has become an issue as studies have shown that only 40% of patients respond to current treatment regimes. Nursing organizations are proactively working to include pain as one of the basic vital signs, along with pulse, temperature, blood pressure, and respiration. Pain has already been included as the fifth vital sign by the Department of Veterans Affairs.
2 In many hospitals, nurses are using pain rating scales as an initial assessment and evaluation tool. Nurses are teaching patients to use a pain scale in which zero is the minimum and ten the maximum.
3 In 1999 the Joint Commission on the Accreditation of Healthcare Organizations (JCAHO) approved revised standards for pain management and aggressive treatment. This has institutionalized pain nationwide as a vital sign for patient management. Currently a common strategy is to take an interdisciplinary approach that would help to recognize, document, and treat pain promptly and effectively.
4 The recommended JCAHO standards are given in the Patient Rights Section One of their official handbook,
The Comprehensive Accreditation Manual for Hospitals. These standards can be used for both staff and patient education.
5The most difficult type of chronic pain to manage effectively is central pain. This type of pain results from injury to the ascending pain pathways and associated structures. Even quite small lesions to these areas can be clinically significant. Central pain has been reported following 3% to 8% of strokes, occurs in 20% to 44% of multiple sclerosis patients, and is recognized with increasing frequency in Parkinson's disease.
6,7 Commonly, patients are referred to psychiatry for evaluation when central pain has gone unrecognized or misdiagnosed as “drug-seeking,” mood disorder, or somatoform disorder. The purpose of this paper is to summarize the complex functional anatomy of central pain and briefly note the therapeutic options for its treatment.
FUNCTIONAL ANATOMY OF PAIN
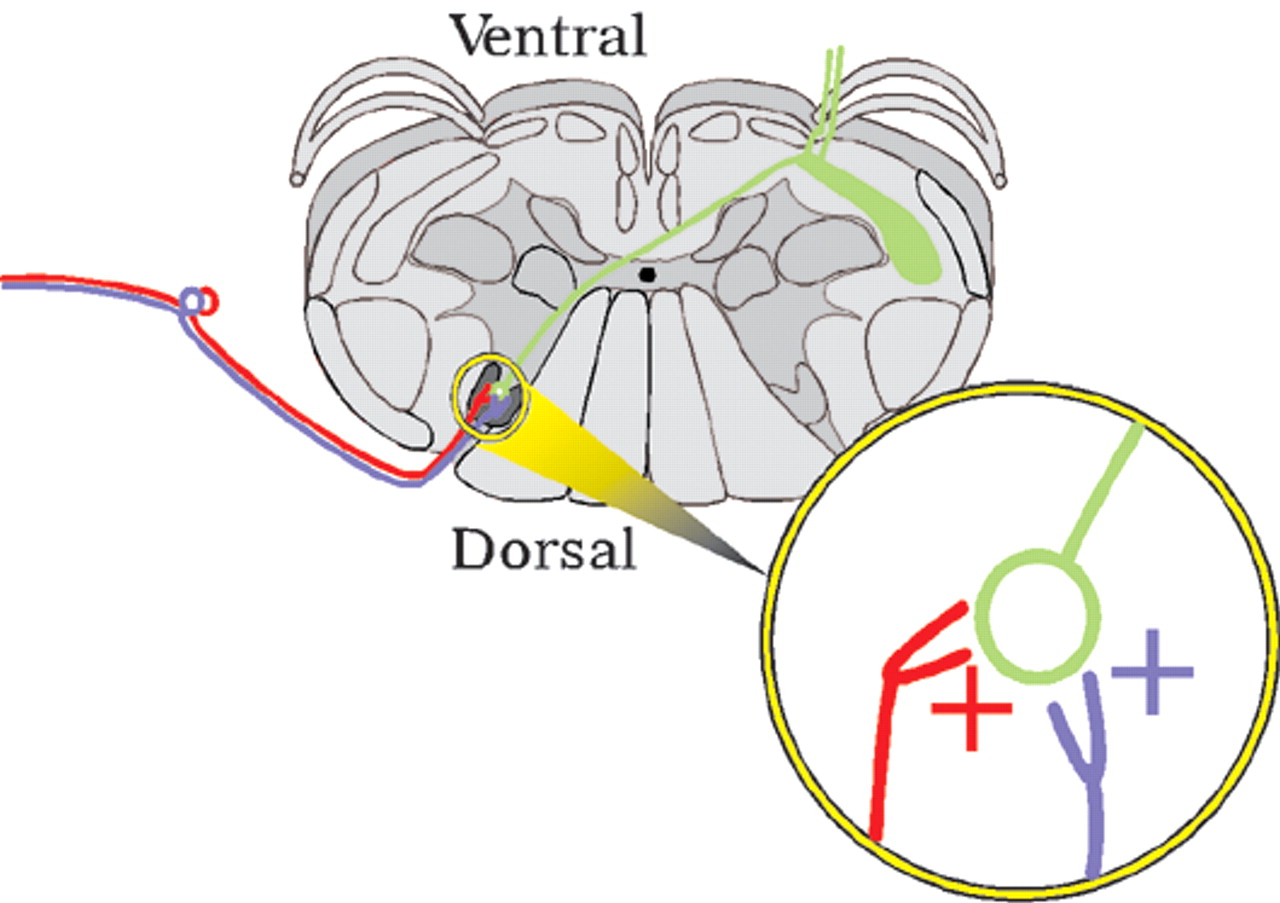
Normal pain (nociceptive pain) results from activation of free nerve endings in peripheral tissues. These nociceptive primary afferents have little or no activity under normal circumstances. Release of inflammatory substances following tissue injury activates A delta nerve endings (small-diameter myelinated fibers that carry pinprick or primary pain) and C nerve endings (small-diameter unmyelinated fibers that carry slow-burning or secondary pain), increasing their sensitivity. This decrease in the response threshold results in increased firing of these afferents in the spinal cord (felt as ongoing pain). Both nociceptive and touch afferents converge on and activate second-order spinal cord neurons, which send projections to the brain via the spinothalamic tract (
Figure 1). This convergent input (and co-release of substance P, which activates neurokinin receptors) causes hyperexcitability in the second-order neurons in the spinal cord. Responses are increased to both nociceptive input (hyperalgesia) and to touch. Light touch in and around the area of injury evokes pain (allodynia). Peripheral tenderness spreads beyond the actual area of injury as a result of this sensitization (secondary hyperalgesia).
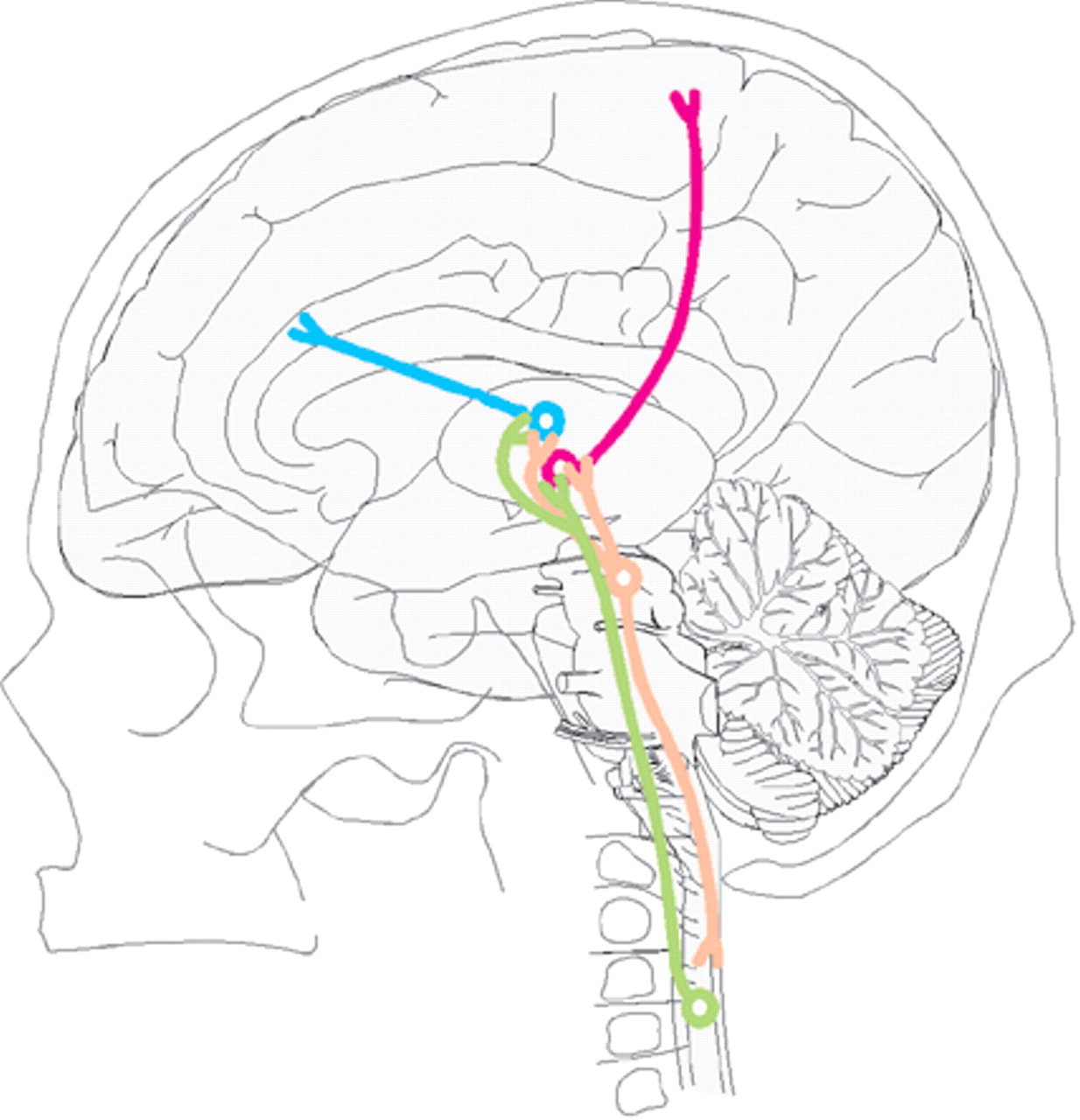
From the spinal cord, pain sensation is relayed to thalamus and brainstem via the spinothalamic and spinomesencephalic tracts (
Figure 2). From the thalamus, the pain system is believed to separate into a lateral system involved in perception of pain (sensory and discriminative) and a medial system involved in emotional coloring of pain (affect and motivation).
7–12 The projection to the primary somatosensory nucleus of the thalamus (ventral posterior complex, VP) is important for perception of pain.
13 VP in turn projects to primary (SI) and secondary (SII) somatosensory cortices as well as the dorsal portion of middle-posterior insular and posterior parietal cortices (
Cover and
Figure 2).
14 The spinothalamic tract also projects to other thalamic nuclei. It is likely that the emotional aspects of pain are modulated in part by the influence of the midline and intralaminar nuclei on the anterior cingulate cortex (Brodmann area 24) as well as anterior insular and orbitofrontal cortex (
Cover and
Figure 2).
14Higher centers also modulate pain via brainstem structures. In particular, the dorsal raphe nucleus in the brainstem projects to both the thalamus and spinal cord to modulate the intensity of pain.
15 The periaqueductal gray receives projections from many limbic, sensory, and autonomic structures. Among other actions, it modulates the activity level of the dorsal raphe nucleus.
16 Functional brain imaging studies using positron emission tomography and functional magnetic resonance imaging have shown activation in somatosensory, prefrontal, cingulate, insular and parietal cortices, thalamus, midbrain, and basal ganglia, in response to painful stimuli.
11,17,18 Receptor mapping and binding studies indicate that many areas included in the medial pain system are also part of the endogenous opiate system.
19 Opiate binding is decreased in some of these structures in patients with central pain, perhaps indicating maximal activation of the endogenous pain control system.
20 This may be why opiates are generally not effective in patients with central pain.
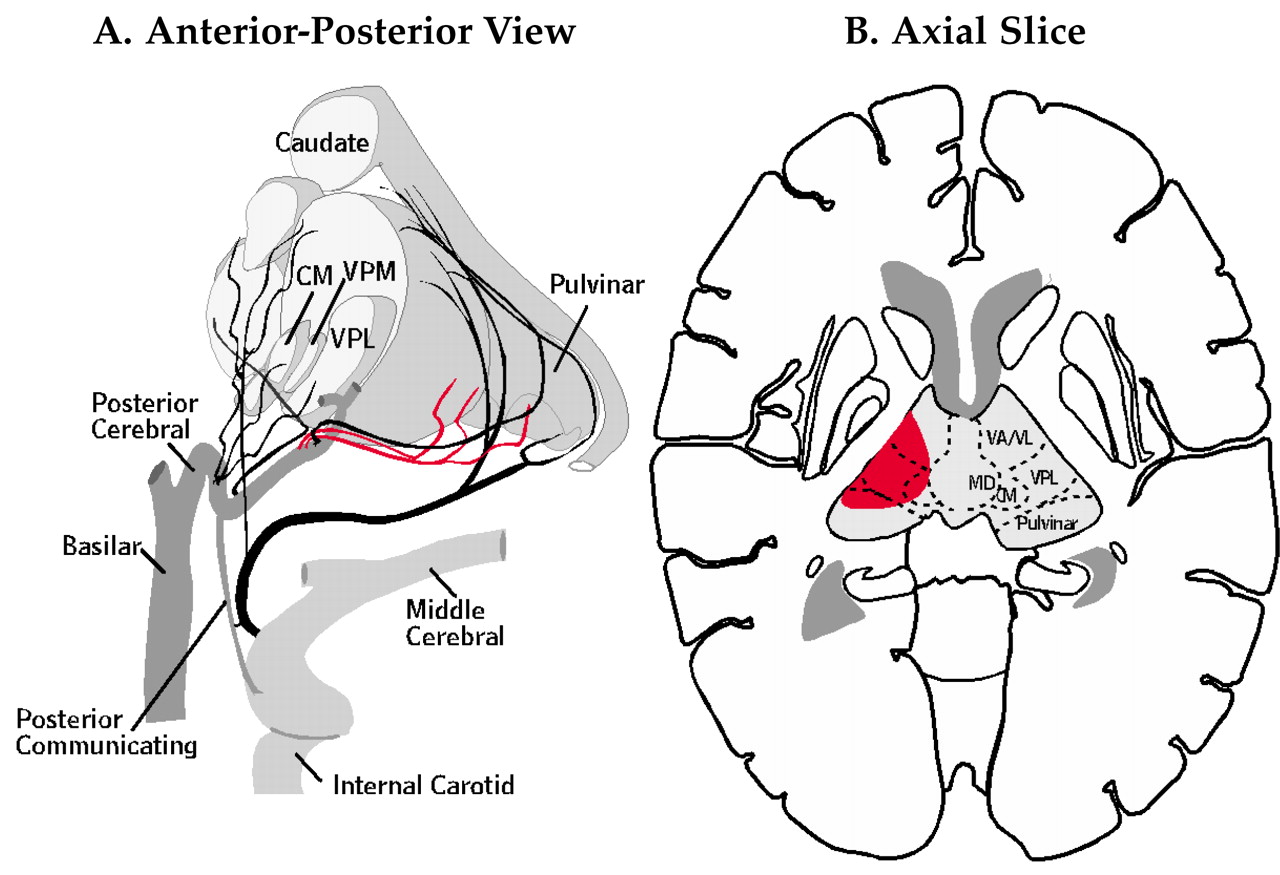
11Central pain is neuropathic pain resulting from damage to portions of the central nervous system involved in perception of pain. Central pain arises most commonly from injury to the ascending spinothalamic tract or VP of the thalamus. It is frequently referred to as thalamic pain (Dejerine and Roussy syndrome;
Figure 3).
21–23 However, because injury to other areas can give rise to central pain, the more general term central poststroke pain (CPSP) has also been used. This term is also too narrow. Although stroke is the most common etiology, central pain can follow injury from many other conditions, such as subarachnoid hemorrhage, compression, traumatic brain injury, abscess, and demyelinating or degenerative processes.
Central pain develops within a week after injury in 35% to 40% of patients. However, its appearance may be delayed by more than a year in as many as 11% of patients.
6,22,24 If the pain is late in onset, its relationship to the initial injury may not be obvious. Thus a detailed clinical history and appropriate neuroimaging are vital. The pain is frequently perceived as arising from the peripheral area affected by the central injury and is felt to be diffuse rather deep or superficial. It is exacerbated by any stimulus or movement of the affected area of the body. Once established, it tends to spread. There is no inflammation in the affected area. A common aspect of central pain is allodynia, in which the sensation of pain is experienced from a nonpainful stimulus. Even brief movement of clothing, for example, can cause severe burning pain.
THERAPEUTIC OPTIONS FOR CENTRAL PAIN
Treatment of central pain can be difficult. To manage these patients effectively, it is very important to recognize and diagnose central pain early because the longer it goes untreated, the harder it is to control.
25 In the last two decades many different approaches have been tried. There is no single method that will reliably and effectively work in all patients.
Medications are successful in some patients, but only a few double-blind studies have been done. Systemic narcotic analgesics are occasionally effective.
26 Tricyclic antidepressants are reported to relieve central pain in more than one-half of patients.
25,27 Addition of mexiletine to amitriptyline may provide pain relief in previously unresponsive patients.
25 Tricyclic antidepressants, however, come with significant drawbacks due to their anticholinergic and sedative properties as well as the small safety margin (lethal in overdose). Carbamazepine may also be useful. Other medications, such as lamotrigine, gabapentin, and nefazodone, also show promise for the treatment of central pain, but at present the evidence is limited to case studies and anecdotal reports.
25,28,29 Initial double-blind studies indicate that the newer generation antidepressants that are specific serotonin reuptake inhibitors are probably not effective.
6 Electrical brain stimulation has been tried in patients in whom medications have failed. Common targets are VP of the thalamus, posterior limb of the internal capsule, and motor cortex, although brainstem stimulation is used in some instances. Deep brain stimulation is reported to be successful in more than half of chronic pain patients overall, but it provides relief to only 20% to 24% of patients experiencing central pain.
30,31 Surface stimulation of motor cortex, in which electrodes are placed in the epidural space over the portion of motor cortex serving the body area where the pain is experienced, is reported to provide relief in 46% to 77% of central pain patients.
26,32 Brain lesioning is the last resort for the treatment of central pain patients. It is seldom used because of the high risk of developing iatrogenic central pain.
33,34 The main targets for this type of treatment are thalamic (VP, medial thalamus, intralaminar nuclei).
33,34 Success rates are difficult to determine, although one series reported 60% (3 of 5) patients benefited from lesion of the centramedian nucleus (one of the intralaminar nuclei).
34