PTSD has been studied from many aspects, including searches for the neurochemical and neuroanatomical changes that occur. This work has produced many conflicting results because of variations in the population studied, study design, outcome measures, and comorbid variables such as preexisting conditions. Newport and Nemeroff provide a review of documented neurobiological abnormalities.
4 Neurochemical abnormalities have been most often reported in the hypothalamic-pituitary-adrenal axis, particularly increased levels of norepinephrine and epinephrine. Patients with PTSD have been found to produce elevated levels of corticotropin-releasing factor but low levels of cortisol. Other possible abnormalities include increased levels of triiodothyronine (T
3) and thyroxine (T
4), serotonin dysfunction, impaired γ-aminobutyric acid (GABA) function, increased cellular immune activation, sensory processing abnormalities, and dysregulation of the endogenous opioid system.
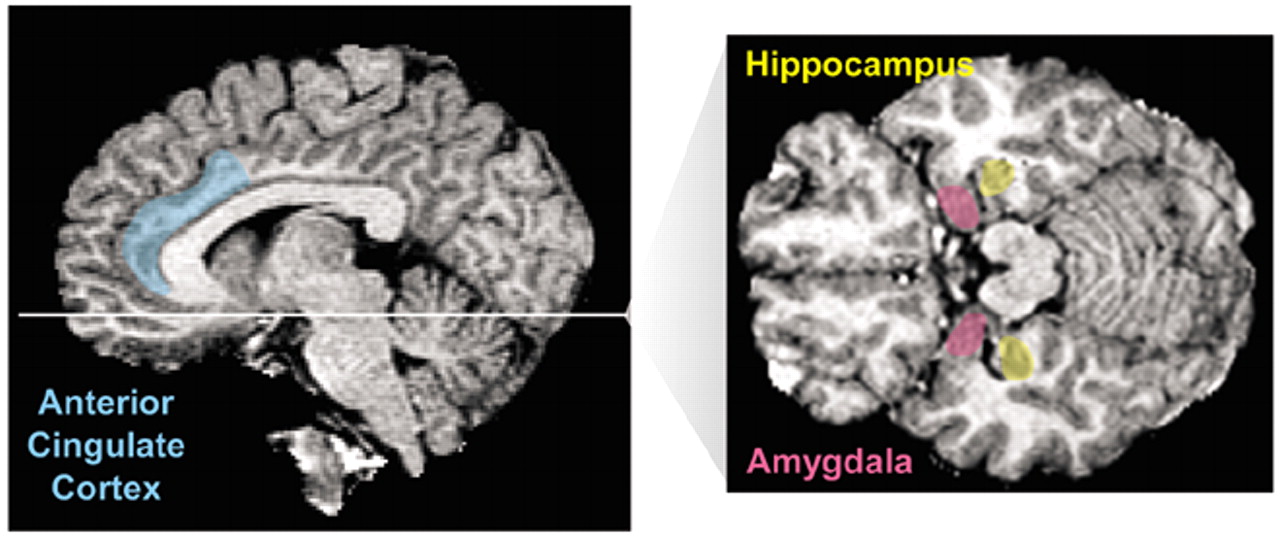
4Study tools have included magnetic resonance imaging (MRI), magnetic resonance spectroscopy, single photon emission computed tomography (SPECT), positron emission tomography (PET), ligand studies with radioisotopes that tag neurotransmitters or medications, and, more recently, functional magnetic resonance imaging (fMRI). Together these studies have examined both brain morphology and brain functioning in subjects with PTSD. Comparison groups have included individuals who have not experienced trauma and subjects with traumatic exposures who did not develop PTSD. Hull has summarized the central findings of these studies; they include decreased hippocampal volume, increased amygdala activity, decreased anterior cingulate cortex activation, decreased Broca's area activity, right hemispheric lateralization, decreased
N-acetyl aspartate in medial temporal regions, and activation of the visual cortex.
5 Theories to explain these findings include the pivotal role of the amygdala in fear conditioning coupled with roles of the anterior cingulate and the hippocampus to extinguish fear, as well as the role of Broca's area in attaching meaning or significance to experiences that can be translated into words.
5,7 Bremner, reviewing both published and unpublished data, noted in addition a significant role for the orbitofrontal cortex and the posterior cingulate.
6 Although these are exciting findings, as noted earlier, replication of results and a clear understanding of the relationships between brain structures is still lacking. Many unanswered questions await further study with both the older and the newer imaging tools. Among the newer tools is fMRI, which combines brain function and matching anatomical images.
Functional Magnetic Resonance Imaging
The principle underlying fMRI is that deoxygenated hemoglobin (deoxyhemoglobin) is paramagnetic and thus acts as a natural MR contrast agent.
8–12 The signal intensity of blood in an fMR image is therefore dependent on the local balance between oxygenated and deoxygenated hemoglobin—hence the term blood-oxygen-level-dependent (BOLD) response in describing the MR technique used to acquire the images. The presence of deoxyhemoglobin within the blood vessel creates a small magnetic field gradient, affecting an area of perhaps 1–2 radii beyond the vessel wall. Numerous different approaches are used to make the MRI sensitive to the presence of deoxyhemoglobin (susceptibility-weighted images).
8,10 One problem with the most commonly used methods of obtaining susceptibility weighting is the presence of susceptibility-related artifacts in areas of magnetic field inhomogeneity, such as the interfaces between brain, bone, and air. Thus regions of importance in neuropsychiatry that are adjacent to bone, such as the orbitofrontal cortex and the inferior temporal region, are difficult to assess.
When an area of brain suddenly becomes more active, such as when it is participating in the performance a cognitive task, the increase in local blood flow is larger than that required to meet metabolic demand. The level of deoxyhemoglobin in the blood decreases, causing a slight (1%–5%) increase in signal intensity in that small area of brain. Although this is too small a change to see in the image by eye, it can be measured when the signal intensity under a baseline (resting) condition is compared with the signal intensity under an activated condition. Thus, unlike PET, in which actual blood flow or metabolic rate can be measured, all fMRI measurements are relative to a baseline condition. Defining and creating a baseline state for comparison is a considerable challenge.
13 For example, if the brain area of interest is abnormally active under baseline conditions, further activation may not be measurable, resulting in an apparent absence of activation when the image sets are analyzed.
Areas of higher signal intensity are presumed to indicate areas of higher neuronal activation. Previously it was assumed that the BOLD response was correlated with action potential generation and thus could be used as a measure of connectivity between activated brain regions. However, several lines of evidence, including acquisition of fMR images at the same time as electrophysiological recording of both neuronal spiking and local field potentials, indicate that this may not be the case.
12,14,15 Rather, the BOLD response correlates best with the local field potential, (which reflects incoming activity and local processing) rather than with action potential generation (output). Changes in local cerebral blood flow are also correlated with local field potentials, not with spike rate.
14 Thus an fMRI activation could be present without an increase in the firing rate of the projection neurons. In addition, an increase in local cerebral blood flow and therefore a BOLD response would be expected for both excitatory and inhibitory processing, because energy demand is increased in both.
There are many ways of analyzing fMRI data.
8,9 The simplest is to subtract the average of the baseline images from the average of the activated images. A more sophisticated approach is to correlate the signal intensity of each voxel with the stimulus condition, identifying voxels in which the signal intensity is highly correlated with the stimulus presentation. Other approaches are also used, including use of a voxelwise
t test or calculation of the hemodynamic response to the stimulus for each voxel. All of these approaches can be spatially filtered to eliminate voxels that appear activated as a result of random noise (not part of a larger group of activated voxels). Neuroanatomical localization is provided by overlaying areas that either are greater than the chosen signal intensity threshold or meet particular statistical criteria onto companion structural MR images obtained during the same session.
There are several methodological issues of importance in fMRI. A major challenge is differentiating areas of increased signal intensity that are within the microvasculature of the parenchyma of the brain and due to brain activity from those that are within slightly larger draining veins that are at some distance from the area of brain activation.
12,16 One approach to this problem is to collect an MR angiogram along with the structural and functional data sets. The locations of angiographically identified vessels are compared with the locations of areas of increased signal intensity in the fMRI data set. Those that coincide can be excluded from further analyses. Another approach is to alter the fMRI acquisition so that it is much more sensitive to the signal from the microvasculature and less sensitive to the signal from larger vessels. Motion artifacts are also a problem in fMRI. Any movement, including minor head movements and movements related to respiration and speech, can create spurious areas of activation or mask areas of true activation.
9 Head restraints and postprocessing are both important in this regard.
8,16The environment of the MR scanner has aspects that are particularly troublesome in neuropsychiatric research. The scanner bore is a long, narrow tube. During imaging, loud noise is created by gradient switching. Thus, the subject is in a very loud, uncomfortable, confined space that is liable to induce a claustrophobic response. Even some control subjects have difficulty tolerating these conditions. This is a substantial problem in neuropsychiatric research, because many of the populations of interest have difficulty remaining perfectly still for long periods. In addition, the physical limitations of the MR environment coupled with the need to prevent motion makes the presentation/response conditions challenging, particularly for the more complex cognitive tasks (as opposed to simple sensory or motor tasks).
fMRI has several advantages over other techniques for functional imaging of the brain. Most important, it is totally noninvasive and requires no ionizing radiation or radiopharmaceuticals. Minimal risk makes it appropriate for use in children as well as adults. Multiple imaging sessions can be conducted with individuals for longitudinal studies. The anatomic resolution of fMRI is higher than in other techniques as well. In addition, the required equipment is widely available. However, fMRI is not easy to implement and analyze, and this may limit its clinical usefulness. At present, it should be considered a research technique.
As noted earlier, a typical fMRI study requires comparison of a baseline with an activated state. In some cases the baseline condition is simply the absence of a specific cognitive task or stimulation condition. In other cases it is a variation on the cognitive task or stimulus condition, such as changing a single variable. The activation can be anything from a simple sensory stimulation to a complex cognitive task. The baseline and activated conditions are usually presented several times in alternating sequence. These repetitions allow averaging of data, thus increasing statistical power and making it possible to analyze data from individuals (as opposed to averaging across a group).
16 However, an underlying assumption is that the brain activations in each repetition occur in the same regions. In some cases this may not be true.
In PTSD research, reminders of the traumatic event are often used as stimuli either to induce PTSD symptoms or to probe the network of brain regions responsible for processing trauma-related information. Although exposure to generic stimuli can be used, use of a script that is individualized for each subject increases the likelihood of a strong reexperiencing. This approach has been criticized because the stimuli do not necessarily affect all subjects to the same degree. The psychological impact may differ across subjects or between groups. If so, differences in brain activation may be due to differing degrees of fear experienced rather than differences in brain processing of fear.
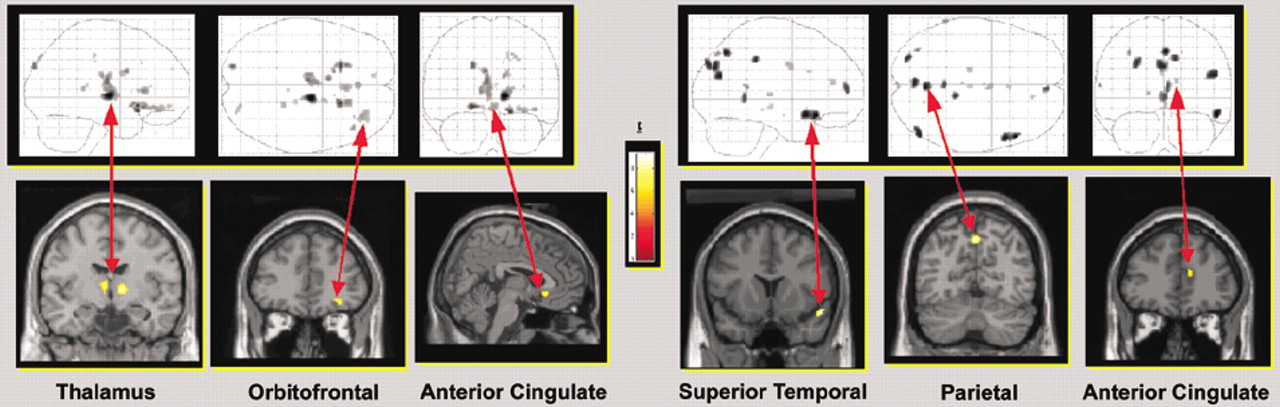
17One group has used script-driven imagery to evoke traumatic memories in conjunction with fMRI measurement of brain activation (
Figure 2).
18,19 In this series of experiments, subjects listened to 30 seconds of their script, then spent 30 seconds remembering the traumatic event as clearly as possible, and then spent 120 seconds relaxing and recovering. This cycle was repeated three times. Baseline images were collected 60 seconds before each period of recollection. Activation images were collected during the final 30 seconds of each period of recollection. The final fMRI data sets were an average of all three cycles. Heart rate was recorded as an indicator of autonomic state.
In their first study the authors reported that patients with PTSD (six whose PTSD was a result of sexual abuse or assault, three because of motor vehicle accidents) showed less activation in the thalamus, medial frontal cortex (Brodmann's area 10/11), and anterior cingulate cortex (Brodmann's area 32) during trauma remembering than control subjects with similar trauma exposure but without PTSD.
18 The PTSD group also had increases in heart rate during remembering, an indication of autonomic reactivity. The authors suggested that higher levels of arousal in the patients with PTSD (as indicated by increased heart rate) may alter thalamic processing, disrupting information flow to the cortex.
In their second study the authors selected patients with PTSD (all seven as a result of sexual or physical abuse) who did not have an increase in heart rate during remembering of traumatic events. The authors noted that approximately 30% of their patients show this type of dissociative response. The PTSD and control groups had similar levels of thalamic activation. The PTSD group had higher activations (predominantly on the right) in the superior and middle temporal gyri (Brodmann's area 38), occipital lobe (Brodmann's area 19), parietal lobe (Brodmann's area 7), inferior frontal gyrus (Brodmann's area 47), medial frontal and prefrontal cortex (Brodmann's areas 9 and 10), and anterior cingulate cortex (Brodmann's areas 24 and 32). The authors noted that activation of the superior and middle temporal gyri are consistent with the temporal lobe theory of dissociation, and activation of frontal areas is somewhat consistent with the corticolimbic model of depersonalization. They also emphasized the importance of categorizing patients with PTSD according to their response to traumatic memories (e.g., hyperarousal versus dissociation), since it is likely that the areas of the brain involved differ.
Another group has used fMRI in conjunction with cognitive tasks designed specifically to activate areas of the brain implicated in PTSD to assess responsivity.
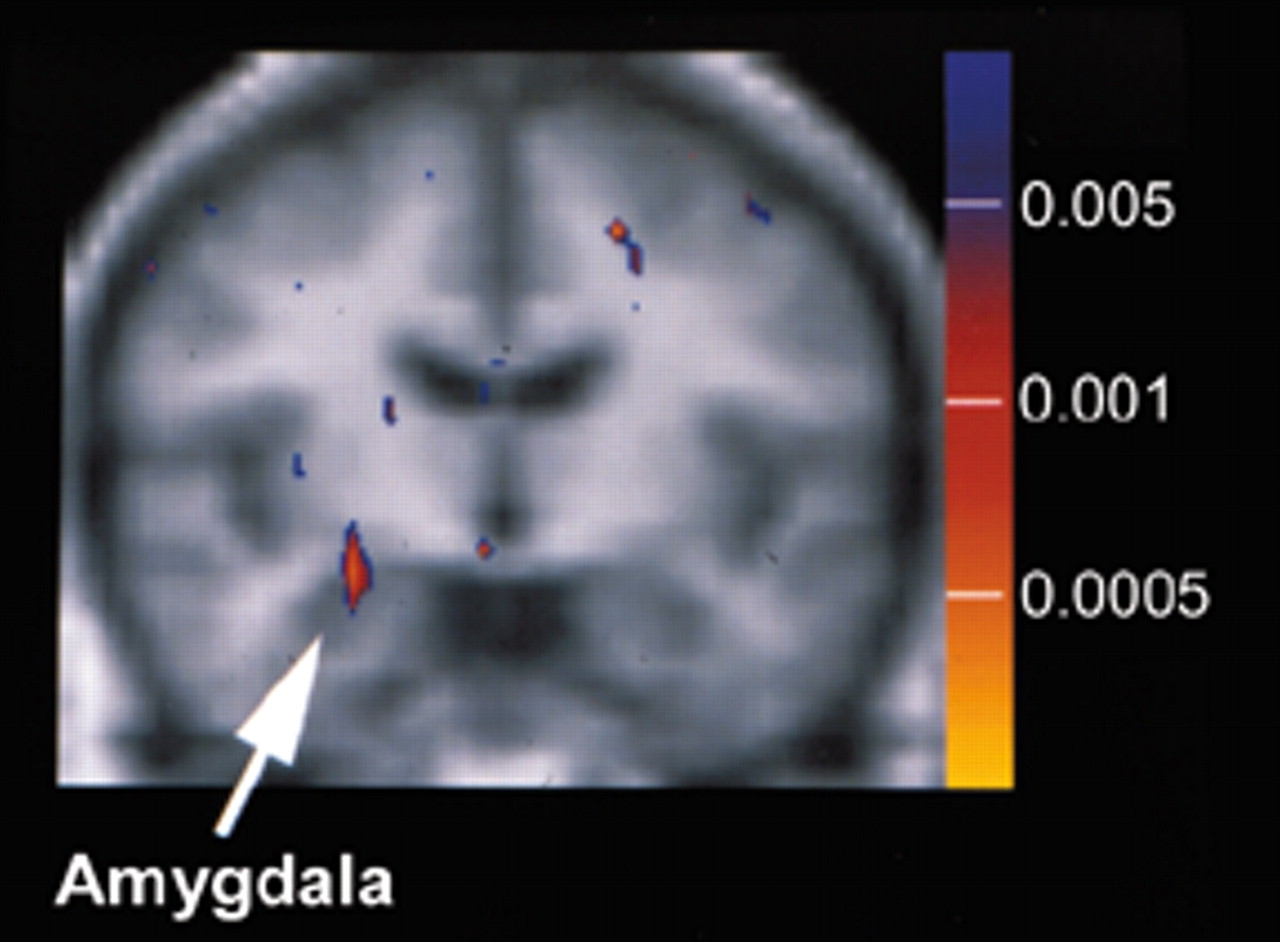
1,20 Both the amygdala and the medial frontal cortex are activated during passive viewing of fearful faces. The group's first study employed a cognitive task designed to probe the responses of the amygdala to fearful faces in the absence of cortical modulation.
1 To accomplish this they presented emotionally expressive faces by the technique of backward masking (masked-faces paradigm). Alternating blocks of 56 masked-fearful, 56 masked-happy, and a fixation condition are shown, with each type of stimulus presented twice per second (for a total of 28 seconds per block). Each of the 56 presentations consisted of a short exposure (33 msec) to a fearful or a happy face (target) followed by a longer exposure (167 msec) to a neutral face (mask). Previous studies have shown that this approach activates the amygdala but not the medial frontal cortex. Eight men with combat-related PTSD were compared with eight men with similar exposure who did not have PTSD. Vascular contamination was minimized by using MR angiography to identify larger vessels and collecting the fMRI with an asymmetric spin-echo sequence to minimize contributions from smaller vessels. Only the areas of the amygdala, medial frontal cortex, and fusiform gyrus were analyzed. As expected, the two groups showed a similar level of fusiform gyrus activation in response to faces, indicating that overall hemodynamic responses were similar. Neither group had medial frontal activation in response to fearful faces. Both groups had amygdala activation in response to fearful faces, but the level of activation was significantly higher in the PTSD group (
Figure 3). In addition, the level of activation in the amygdala was correlated with PTSD symptom severity but not with severity of trauma exposure. The authors noted that this protocol distinguished the two groups with 100% specificity and 75% sensitivity.
In a second study, the same group measured the responsivity of the rostral anterior cingulate cortex.
20 This brain region is thought to have a role in the processing of emotional stimuli, and it has been shown to be activated in normal individuals during performance of an emotional variant of the Stroop task, in which emotionally negative words are viewed and counted (as compared with neutral words). Previous studies have shown that individuals with PTSD have slower response times to trauma-related negative words in this task (as compared with general negative words). Eight men with combat-related PTSD were compared with eight men with similar exposure who did not have PTSD. Blocks of neutral words alternated with blocks of general negative words and blocks of trauma-related words, all blocks 30 seconds in duration. For each trial, subjects viewed (for 1.45 sec) a set of one to four identical words and were required to press a button to indicate how many words were displayed. Comparisons were made of response times, error rates, and areas of activation to neutral, generally negative, and trauma-related words.
The PTSD group had slower response times and made more errors in response to all three types of words. Activation of the insular cortex was found in both groups. Unlike the control group, the PTSD group did not have significant activation of the rostral anterior cingulate region. The authors noted that this finding is consistent with the hypothesis that PTSD involves a failure of medial frontal regions to properly inhibit the amygdala.