Parkinson's disease (PD) is the second most common neurodegenerative illness in the United States, affecting between 500,000 and 1 million individuals and accounting for costs of up to 20 billion dollars in the United States.
1 Most, but not all, cases begin after the age of 50, with the prevalence increasing from 1.5% to 2.5% among people over 70 years of age.
2 The illness is progressive and leads to significant functional disability.
3Depression is the most common neuropsychiatric disturbance found in PD. The reported prevalence of depression varies, depending on study methodology, but a figure of approximately 40% is generally accepted, with one-half of the patients meeting criteria for major depression and one-half meeting criteria for dysthymia.
4 Depression is particularly important in these patients because, in addition to the personal suffering, it is associated with faster progression of physical symptoms,
5 greater decline in cognitive skills,
4 and greater decline in ability to care for oneself.
4–6Anxiety is another common and disabling nonmotor complication of PD. A number of studies have examined this issue. Menza et al.
7 found that 28% of patients with PD had a formal DSM-III-R anxiety disorder diagnosis and another 40% had anxiety symptoms. Furthermore, depression is highly comorbid with anxiety in these patients. Of patients with PD who have a diagnosis of depression, up to 67% also have an anxiety disorder.
7 While it is not clear what impact anxiety has on long-term issues in PD, it has been associated with poorer long-term outcome in depressed non-PD populations.
8 Furthermore, anxiety that is comorbid with depression in non-PD patients is effectively treated with antidepressants, which may improve long-term outcome.
9Despite the importance of depression, anxiety, and disability in PD, there are virtually no empirical data from well-designed controlled studies that can direct treatment. There are a number of older controlled trials of depression with tricyclic agents, but each of these is so methodologically compromised as to render the results nongeneralizable.
10–12 There is also a small controlled study of bupropion,
13 but it examined only 12 depressed patients, of whom 5 improved. Therefore, it is obvious that these studies are very limited in the extent to which they can inform clinical practice. While there are open label trials supporting the use of sertraline,
14,15 paroxetine,
16–18 fluoxetine,
18 and fluvoxamine
18 for the treatment of depression in PD, none of these trials assessed the important outcomes of anxiety, disability, and cognition.
There have been two open-label studies of citalopram in depressed PD patients. A study conducted by Rampello et al.
19 had 18 patients, 15 with dysthymia and three with major depression, of whom 15 improved, though no details on the amount of improvement are given. Additionally, this study does not assess other important outcomes such as disability, anxiety, and cognition. In a study by Dell'Agnello et al.,
18 62 patients were given fluoxetine, fluvoxamine, sertraline, or citalopram (15 patients). Dell'Agnello et al.
18 reported that a significant improvement in depressive symptoms from baseline to the end of the study was achieved with all of the selective serotonin reuptake inhibitors (SSRIs), without any difference between the four drugs. They did not, however, provide details of the degree of improvement on the depression measure, nor did they measure the outcomes of anxiety, cognition, and disability.
There is one controlled trial of an SSRI, citalopram,
20 which found no difference between active drug and placebo, but there are significant concerns about the methodology of this study. A subtherapeutic dose of citalopram was used, depression was rated only at 0 and 6 weeks, and the authors seemed to indicate that many of the patients they entered into the study did not, in retrospect, have major depression. There also was no attempt to measure the outcomes of disability, anxiety, and cognition.
As there are no treatment trials in patients with PD that assess the impact of treating depression on anxiety, disability, and cognition, an attempt to provide some preliminary data on this issue is important. We therefore undertook a prospective, open-label trial of citalopram in carefully diagnosed patients with PD to include an examination of the impact of treatment of depression on anxiety, disability, and cognition. We chose to examine citalopram, the most potent of the SSRIs, as it is now widely used as an antidepressant and it does not have anticholinergic properties or significant P450 interactions with other medications used in PD and other medical disorders.
21METHODS
Ten consecutive patients with idiopathic, levodopa-responsive PD were recruited from the Robert Wood Johnson Medical School Movement Disorders Clinic and the local community. The study was approved by the Robert Wood Johnson Medical School Institutional Review Board. All patients had a clinical diagnosis based on DSM-IV
22 criteria of major depression and a 21-item Hamilton Depression Rating Scale
25 (HAM-D) score of 18 or greater. Patients were excluded if they had any Axis I diagnosis other than a depressive or anxiety disorder, including any current (within 3 months) diagnosis of alcohol or substance abuse/dependence (with the exception of nicotine dependence). If patients were currently on psychotropic medications, including antidepressants, at the time of initial screening, there was a 1-week washout period (except for fluoxetine, which had a 4-week washout period) prior to entering the study. Patients on deprenyl were excluded as were patients with a known history of nonresponse to citalopram or nonresponse to more than one trial of an adequate dose and length of an approved antidepressant. Patients with a Mini-Mental State Examination
24 (MMSE) score of less than 20 were also excluded.
After signing informed consent, patients were given flexible doses of citalopram, beginning at 10 mg/day, in an 8-week, open-label study. Patients were seen at 2-week intervals. The primary outcome measure was the score on the HAM-D, and secondary outcome measures included scores for anxiety (Hamilton Anxiety Rating Scale
25 [HAM-A]), PD symptoms (Unified Parkinson's Disease Rating Scale
26 [UPDRS]), functional impairment (Rapid Disability Rating Scale
27 [RDRS]), and cognition (MMSE). Adverse events were elicited by direct questioning.
All analyses were based on last observation carried forward (LOCF) data, and within-group pre-post changes were compared with repeated measures analysis of variance (ANOVA) using SAS PROC (procedure) ANOVA. Cohen's effect sizes (ES) were calculated using pooled standard deviations.
RESULTS
Ten patients (six women, four men) who were taking a mean of 720 mg of levodopa and whose mean age was 67 years were entered into the trial. Eight patients completed the trial. Five patients were taking dopamine agonists, and 3 were also taking catechol-O-methyltransferase inhibitors.
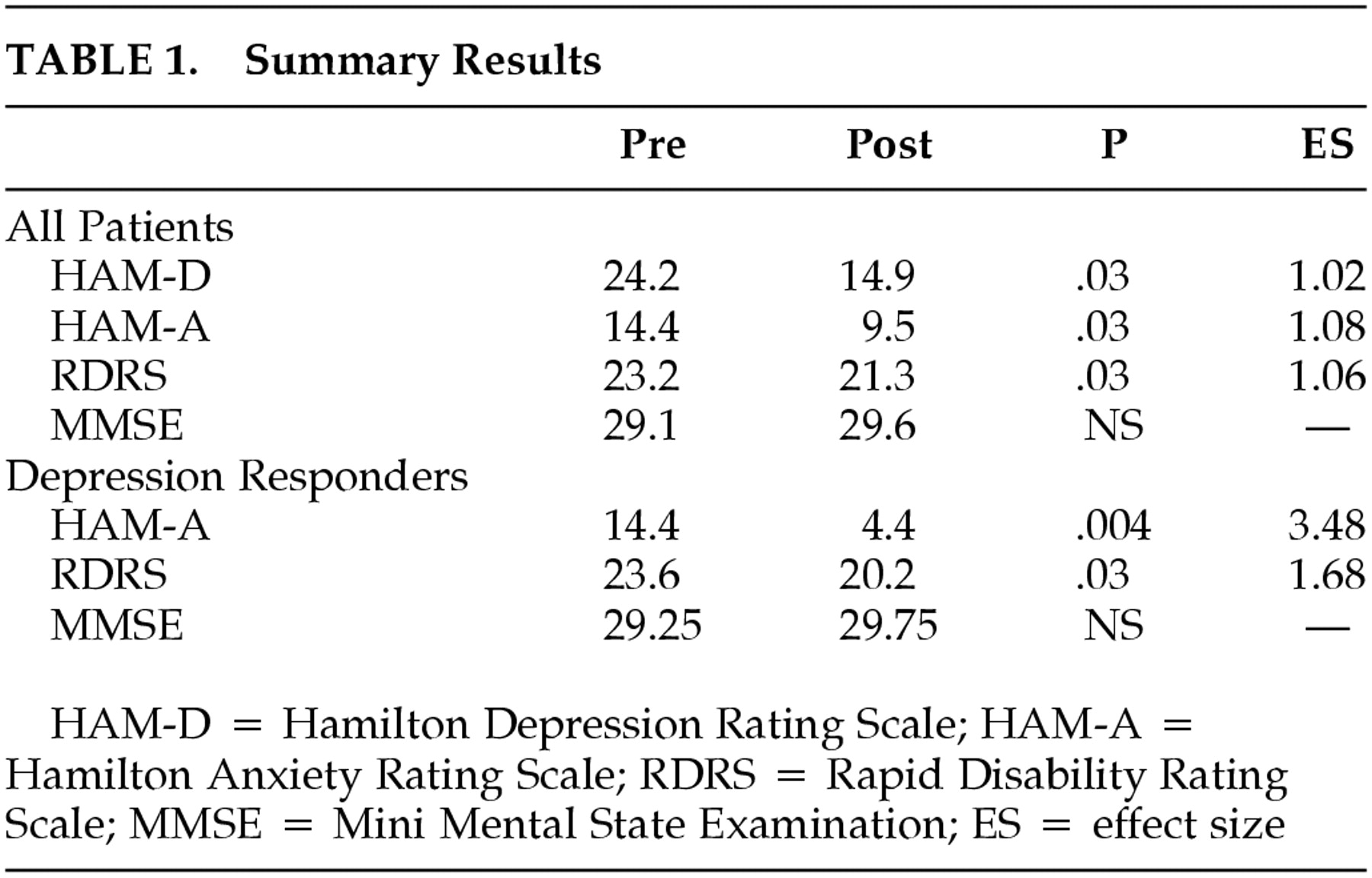
Summary results are presented in
Table 1. The mean baseline HAM-D was 24.2 and improved to 14.9 at endpoint (
F=5.22,
P=0.03, ES=1.02). Eight (80%) of the 10 patients improved on the HAM-D and five (50%) of the 10 were responders based on a 50% or greater improvement in the HAM-D. Strict criteria for remission (HAM-D ≤8) were met by 40% of the patients.
The mean HAM-A at baseline was 14.4 and decreased to 9.5 at endpoint (F=5.7, P=0.03, ES=1.08) in the group as a whole. The RDRS at baseline was 23.2 and declined to 21.3 at endpoint (F=5.63, P=0.03, ES=1.06). The mean UPDRS was 38.6 at baseline and declined to 35.2 at endpoint (P=n.s.), and the mean MMSE improved from 29.1 to 29.6 (P=n.s.). The final dose of citalopram was a mean of 19 mg/day (three patients on 10 mg, six on 20 mg, and one on 40 mg/day).
Responder Analyses
In the 50% of patients who were depression responders (≥50% improvement in the HAM-D), there was a statistically significant decrease in anxiety (HAM-A—F=34, P=0.004, ES=3.48) and a statistically significant improvement in disability (RDRS—F=7.03, P=0.03, ES=1.68). Motor dysfunction (UPDRS) and cognition (MMSE) did not change significantly in this group of patients from baseline to endpoint.
In the 40% of patients who met criteria for depression remission (≤8 on the HAM-D), there was a statistically significant improvement in anxiety (HAM-A—F=17.34, P=0.005, ES=2.95) and disability (RDRS—F=7, P=0.03; ES 1.79). Motor dysfunction (UPDRS) and cognition (MMSE) did not change significantly in this group of patients from baseline to endpoint.
Tolerability
Seven (70%) of the 10 subjects reported an adverse event, but these were generally mild and there were no serious adverse events. One patient withdrew after 2 weeks of drug treatment because of worsening depression, and one patient withdrew after 4 weeks because of persistent nausea and worsening of motor dysfunction. Both were included in the LOCF analysis. The most common side effects were sedation (n=5), GI discomfort (n=2), anxiety (n=2), and dry mouth (n=2). These were generally mild and resolved over the course of the trial.
DISCUSSION
Despite the lack of well-controlled trials of depression treatment in patients with PD, there appears to be a clinical consensus that antidepressants are useful for these patients. A survey of physicians in the Parkinson Study Group
28 found that 26% of their patients with PD were on antidepressants for depression. Fifty-one percent of the physicians used the SSRIs as their first line therapy, and tricyclic antidepressants were used as first-line therapy by 41% of the physicians. While there are no clinical trial data whatsoever on the impact of treatment of depression on anxiety, disability, and cognition, this small, prospective study provides some preliminary data on these questions.
In this open-label, prospective trial of citalopram, depression scores improved significantly from baseline to endpoint. Eighty percent of the patients improved on the HAM-D, and 50% were responders based on a 50% or greater improvement in the HAM-D. Strict criteria for remission (HAM-D ≤8) were met by 40% of the patients. These results are similar to other open-label trials of SSRIs in depressed PD patients.
This is the first study to examine the response of anxiety to an SSRI in depressed PD patients. For the whole group, the mean HAM-A at baseline was 14.4 and improved to 9.5 at endpoint. While this is a statistically significant improvement, the clinical significance of this is not clear, as most treatment trials of anxious patients require higher baseline anxiety scores. On the other hand, the effect size was large, suggesting that this may be an important response. For depression responders and remitters, the decrease in the anxiety score from baseline to endpoint was statistically significant and numerically large (a change of 10 points in responders and 9.75 points in the remitters). This suggests that treatment of depression in patients with PD can have a beneficial effect on comorbid anxiety.
The presence of depression has been shown to worsen the disability in PD patients,
4–6 and we found in this trial that treatment of depression was associated with small but statistically significant improvements in the RDRS disability measure. This significant improvement in disability was also found, not surprisingly, in the group of depression responders and remitters. While the numerical change was small, small changes in disability may be clinically meaningful. These data suggest that the treatment of depression in patients with PD may improve functional disability as measured by the RDRS.
There are a number of case reports suggesting that the SSRIs may worsen the motor signs of PD.
29 This worsening usually takes the form of a general increase in parkinsonian signs. In this study, however, we found that there was no significant change in the UPDRS from baseline to endpoint, suggesting that treatment of depression with citalopram does not, in general, worsen the motor signs of PD.
Parkinson's disease is associated with subtle but widespread cognitive impairment, even in the absence of clinically apparent cognitive decline.
30 Dementia, typically of a subcortical type, also occurs frequently in PD patients.
31 Depression has been correlated with a faster decline in cognitive function in PD patients, and it is therefore reasonable to question whether treatment of depression might improve this dysfunction. The patients in our study were not significantly impaired at baseline (MMSE=29.1), so we were not able to test the hypothesis that treatment of depression can improve cognitive impairment in these patients.
This was a small, prospective, open-label study, with a number of limitations. As with all nonrandomized, open-label trials, many nonspecific factors may have influenced the results. The small number of subjects limits the power to detect differences in subgroup analyses, and the potential biases in patient referrals to a tertiary research center limits the generalizability of the results.
CONCLUSIONS
Despite the limitations inherent in a small, open-label trial at a tertiary referral center, our findings indicate that PD patients are able to tolerate citalopram, and improvements in depression are associated with the use of citalopram. Further, our results suggest that the treatment of depression in patients with PD may improve disability and comorbid anxiety. We also found statistically nonsignificant improvement in motor functioning and cognition in a small sample, which suggests that one might see important changes in these areas of symptomatology in a larger trial. Our study also indicates that larger, controlled trials examining these issues would be informative.
ACKNOWLEDGMENTS
This study was supported by Forest Laboratories.