Cerebellar disorders typically manifest with ataxia—incoordination of movement, instability of gait, impairment of articulation, and difficulty with eye movement and swallowing. It has become apparent recently that many cerebellar patients also experience changes in intellect and mood. Further, mounting evidence suggests that cerebellar pathology may be associated with alterations principally in mental function, rather than motor performance. There is a new realization that defining and understanding the role of the cerebellum in higher order behavior has the potential to benefit patients with cerebellar diseases by suggesting optimal therapeutic and rehabilitation strategies. This paper surveys the current understanding of the cerebellar role in cognitive and psychiatric disorders. To place the consideration of the cerebellum in a larger context, however, we first begin with a description of the cerebellar motor syndrome and a survey of the more common cerebellar diseases.
THE CEREBELLAR MOTOR SYNDROME
The historian Max Neuburger (1868–1955) credits the cerebellum as the structure that catapulted science into the era of the hypothesis-driven experiment.
1 Thomas Willis (1621–1675) asserted that cerebellum was the seat of vegetative functions and critical for survival. This conclusion was most likely derived from the damage produced to the brainstem (medulla in particular) inflicted on awake animals by the techniques used at that time. Nicolaus Steno (Niels Stensen, 1638–1686) challenged Willis' conclusion that damage to cerebellum was incompatible with life, and performed his own experiments on cerebellum that disproved this hypothesis. In this manner, Steno's experimental disproof of Willis' hypothesis initiated the field of experimental physiology and structure—function correlation in neuroscience. Whereas Franz Josef Gall (1758–1828), arguably the founder of cognitive neuroscience, believed that cerebellum was the seat of sexual function,
2 Luigi Rolando (1773–1831) concluded that it was a motor structure, and Marie-Jean-Pierre Flourens (1794–1867) determined that its role was to coordinate movements. The experimental work of Luigi Luciani,
3 David Ferrier and William Aldren Turner,
4 and J.S. Rissien Russell
5 demonstrated motor incoordination in monkeys following cerebellar lesions. The clinical features of the now well-established cerebellar motor syndrome were defined by clinicians including Sanger Brown,
6 Pierre Marie,
7 Joseph François Felix Babinski,
8 and Gordon Holmes.
9The cerebellar motor syndrome thus identified is characterized in contemporary terms as impairment of gait (ataxia), extremity coordination (dysmetria), disordered eye movements, poor articulation (dysarthria), impaired swallowing (dysphagia), and tremor. The basic deficit common to the motor incapacity is impairment of rate, rhythm, and force of contraction. In the early stages of cerebellar degenerative disorders, balance is poor, and there is inability to stand on one leg or perform tandem gait. As the condition progresses, walking is characterized by widened base; turning is problematic and can result in falls; and there is high stepping, staggering, and lurching from side to side. When ataxia is severe, individuals are no longer able to stand or walk without great assistance and effort. Dysmetria of the extremities is evident in dysdiadochokinesis (the impairment of alternating movements), dysrhythmic tapping of feet or hands, terminal dysmetria and swerving of the arm with finger to nose testing, side-to-side dysmetria and proximal overshoot with the heel to shin test, and decomposition of movement evident in the attempt to draw an imaginary circle in the air with the legs. The rebound phenomenon occurs (overcorrection of passive displacement of the limb), as well as overshoot of the affected extremity when following a stimulus rapidly, and sometimes tremor of extremities, head and trunk (titubation). Tone is decreased, a sign formerly thought to be the pathophysiologic basis of the motor disability. Abnormal eye movements at rest include square wave jerks, microsaccades, and chaotic movements termed opsoclonus and ocular flutter. The hallmark feature of nystagmus is present with horizontal and, less often, with vertical gaze. There are saccadic intrusions into the smooth pursuit reflex, hypometric or hypermetric saccades, slowing of saccades, impairment of the normal oculokinetic nystagmus, and loss of the ability to cancel the vestibulo-ocular reflex (as in focusing on a stationery object when the background is moving—performed with the patient rotating about an axis). Speech is characterized by “scanning” dysarthria, with alteration in rate (slower), rhythm (irregular), and force (variable volume). There is slurring of speech, tremor of the voice, and ataxic respiration. When the cerebellar motor syndrome is fully manifest it is a striking and potentially severely disabling condition. As discussed below, the cerebellar motor syndrome does not necessarily occur in all cerebellar diseases. Indeed, in monkeys, and humans with parkinsonian tremor,
10 lesions of the cerebellar dentate nucleus do not produce motor disability.
DISEASES OF THE CEREBELLUM
A large number of diseases involve the cerebellum. A comprehensive consideration of these disorders is beyond the scope of this report, but many of these are listed in
Table 1, and are dealt with elsewhere.
11Hereditary cerebellar degenerative diseases, in particular, have received considerable attention in recent years, as the autosomal dominant spinocerebellar ataxias (SCAs) have been identified as a distinct group of disorders that in many cases have a definable genetic basis. Margolis
12 has simplified the increasingly complex set of spinocerebellar ataxias by classifying them into three discrete groups based on pathogenesis. The polyglutamine disorders, SCAs 1, 2, 3, 7, and 17, result from proteins with toxic stretches of polyglutamine. The channelopathies, SCA 6 and episodic ataxia types 1 and 2 result from disruption of potassium or calcium channel function. And the gene expression disorders, SCAs 8, 10, and 12, result from repeat expansions outside of coding regions that may quantitatively alter gene expression. The remaining SCAs 4, 5, 11, 13–16, 18–22, and 25 are presently of unknown etiology, and do not yet fit into one of these three groups.
The clinical features that potentially differentiate the genetic ataxias from each other are often unreliable, as they may be inconsistent in a given SCA, or are shared by more than one of the SCAs. This is further compounded by the recognition that Friedreich's ataxia, the autosomal recessive GAA triplet repeat disorder on chromosome 9, was formerly considered to be a disease of childhood with an unmistakable phenotype of ataxia, areflexia, extensor plantar responses, neuropathy, scoliosis and cardiomyopathy. Friedreich's ataxia is now know to present well into adulthood (late-onset Friedreich's ataxia [LOFAR]) or have a phenotype marked only by ataxia and preserved or brisk deep tendon reflexes (Friedreich's ataxia with retained reflexes [FARR]). Moreover, the diagnostic challenge to the clinician is complicated by the knowledge that cerebellar features may be principal manifestations of a number of other system diseases, including mitochondrial disorders.
NONMOTOR ASPECTS OF CEREBELLAR FUNCTION
Based on anatomic, physiologic, and clinical information, investigators such as Ray Snider,
13 Robert Dow,
14 and Robert Heath
15 suggested that the cerebellum is a great modulator of neurologic function, and that whatever it does to motor control, it also does the same thing to other kinds of behaviors. Watson
16 discussed the relevance of the cerebellum to higher order function, Frick
17 explored how the vestibulocerebellum was related to the ego, and S.R. Snyder
18 addressed its role in schizophrenia. The incorporation of the cerebellum into the distributed cortico-subcortical neural system subserving cognition was more formally developed as a concept by the Leiners with Dow
19 based on the expansion of the neocerebellum and dentate nucleus along with the cerebral association areas; by Schmahmann and Pandya
20 and Schmahmann
21 based on earlier physiology and clinical observations, and the associative corticopontine projections to the basilar pons in the monkey; by Strick et al.
22 based on the feedback from cerebellar neodentate nucleus to the prefrontal cortex; and by a number of subsequent investigators.
Since the earliest days of investigation into cerebellar function, the possibility that the cerebellum is involved in areas of neurological processing beyond motor control has been raised explicitly. Around the time that Flourens concluded that cerebellum is a motor control device, and long before the work of the behaviorists and clinicians that firmly entrenched the notion of cerebellum as a motor apparatus, investigators (perhaps starting with Combettes
23) began to report that cerebellar pathology was associated with clinical manifestations outside of the motor domain. Reports throughout the 1800s described individuals with different forms of cerebellar damage, including failure of development (agenesis) and cerebellar atrophy, who demonstrated impairments of intellectual function and emotional or psychiatric disturbances.
21 Later clinical studies identified a relationship between the cerebellum and personality, aggression and emotion,
24 and they linked psychosis—schizophrenia in particular—with enlargement of the fourth ventricle, smaller cerebellar vermis, and cerebellar atrophy.
25,26 In the middle and later decades of the 20th century, before the genetic basis of the ataxias was identified and multiple systems atrophy was classified with the synucleinopathies,
27 patients with cerebellar cortical atrophy and what was known as olivopontocerebellar atrophy were found to have cognitive problems. These included impairments in verbal intelligence, visual-spatial abilities, learning and memory, and frontal system functions.
28–31 Tests showed deficits in strategy formation
32 and procedural learning.
33Now that genetic diagnosis of many of the spinocerebellar ataxias is available, new information suggests that there is some degree of cognitive change at different stages in most of the SCAs,
34 although the pattern of neuropsychological deficits has not yet been shown to distinguish between these disorders. Impaired executive functions, deficits in verbal short-term memory, and mild, generalized cognitive impairment
35 have been documented in SCA 1 (CAG repeat on chromosome 6). Patients with SCA 2 (triplet repeat on chromosome 12) may develop poor memory, concentration problems, impairments of conceptual reasoning, and frontal-executive dysfunction on tests of verbal fluency and Luria's test of motor set switching, as well as emotional instability and impulsivity.
36,37 Individuals with Machado-Joseph disease or SCA 3 (CAG expansion on chromosome 14) demonstrate deficits in visual and verbal attention, verbal fluency, and planning and strategy tests such as the Wisconsin Card Sort Test.
38In SCA 13 (chromosome 19), moderate mental retardation (IQ 62–76) occurs in association with a progressive childhood-onset cerebellar “motor” syndrome and developmental delay.
39 Dementia is part of the diagnosis in SCA 17 (dementia, psychosis, extrapyramidal feature and seizures, with a CAG repeat on chromosome 6,
40,41) and in SCA 21 (ataxia with hyporeflexia, akinesia, rigidity, and cognitive impairment in a French kindred, with linkage to chromosome 7).
42 In Friedreich's ataxia the major pathology is located outside cerebellum and the extent of neuropsychological impairment has been studied with varying results. Some have noted impairments on tests of visual-perceptual and visual-constructive abilities, slowed information processing speed, decreased sustained attention, reduced verbal span, deficits in letter fluency, and impaired acquisition and consolidation of verbal information.
43 In contrast, other studies have shown patients with Friedreich's ataxia to be relatively cognitively intact.
44Pathological features are not confined to the cerebellum in most of these hereditary ataxias, and so the cognitive and emotional impairments are unlikely to be related exclusively to cerebellum.
In patients with focal cerebellar lesions, language problems include agrammatism,
45 decreased verbal fluency,
46 and inability to detect one's own errors in tasks such as the verb-for-noun generation paradigm.
47 Patients with ataxia have difficulties with attentional modulation,
48 motor skill learning,
49–51 and the ability to acquire conditional associative reflexes.
52 Experimental studies in animals reveal that electrical potentials are activated in cerebellum by stimulation of somatosensory, visual, and auditory cortices;
53 stimulation of deep cerebellar nuclei evokes autonomic responses,
54,55 grooming, predatory attack, and sham rage;
56,57 and lesions of the cerebellar anterior interpositus nucleus prevent or abolish conditional associative learning tasks.
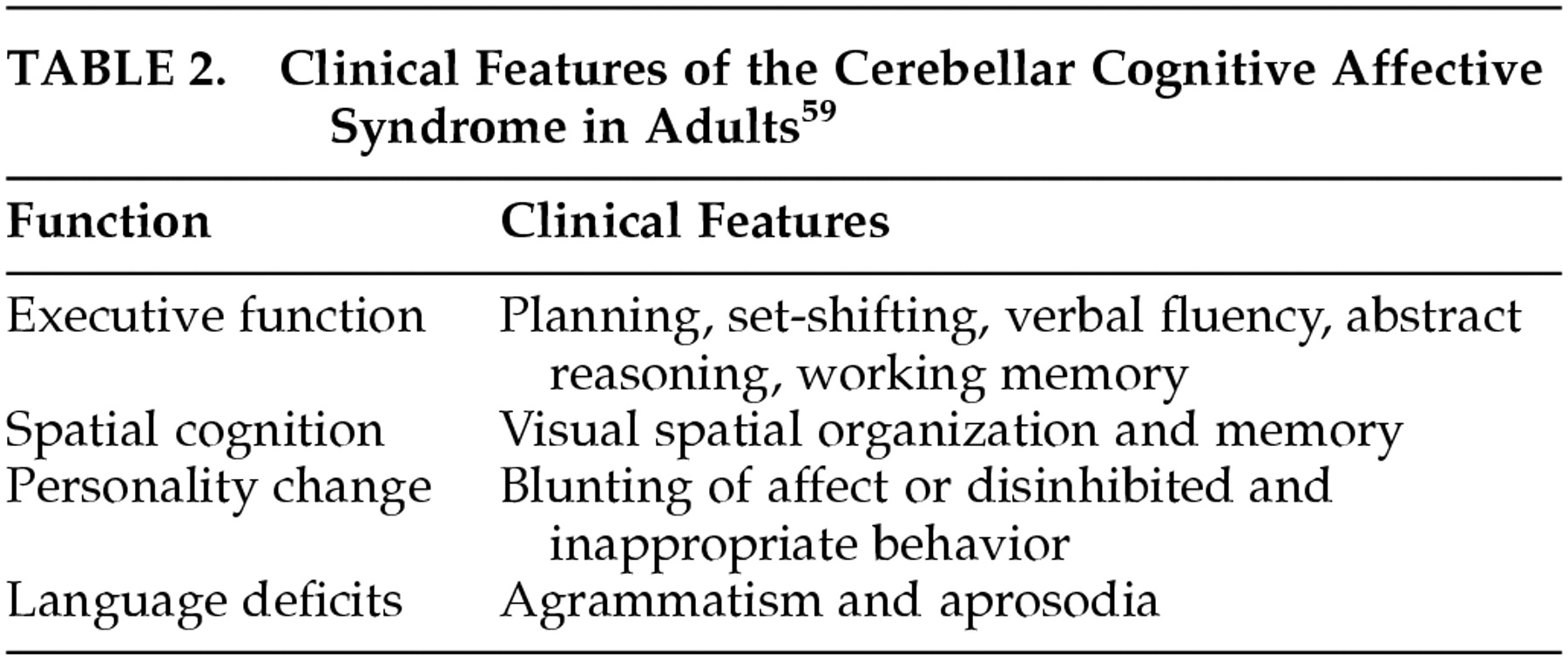
58This clinical and experimental background notwithstanding, a persistent set of clinical questions has limited the consideration of the cerebellum as an integral component of the circuits that subserve cognition and emotion. That is, are the reported cognitive impairments in cerebellar patients observed only with subtle neuropsychological tests, or are they indeed relevant for patients' lives? Do the deficits result from cerebellar damage itself, or from lesions in other brain regions affected by the neurodegenerative disorders?
DYSMETRIA OF THOUGHT AND THE UNIVERSAL CEREBELLAR TRANSFORM
The attempt to understand how cerebellum may be involved in all these higher order brain functions is facilitated by a consideration of the anatomy of the cerebellum and its connections with the cerebral hemispheres and the brainstem. The microscopic anatomy of the cerebellar cortex is quite uniform,
102 and it may therefore seem puzzling that the cerebellum could be involved in all these different aspects of neurologic and psychological function. Anatomic tract tracing studies indicate that there are pathways linking the cerebellum with autonomic,
103,104 limbic,
105 and associative regions of the cerebral cortex
106,107 as well as with sensorimotor cortices. This allows the cerebellum to communicate with brain areas concerned with instinctive behaviors, mood, and the highest levels of cognition and reasoning. Together with Deepak Pandya, we extended the work of earlier anatomists by demonstrating that the association areas in the prefrontal cortices, posterior parietal lobes, superior temporal regions, and parahippocampal areas, send information in a precisely organized manner to the nuclei of the basilar pons,
20,106–110 from where the information is conveyed to the cerebellum. Middleton and Strick
22 demonstrated that the deep cerebellar nuclei, notably the dentate, send information back to those areas of the prefrontal cortex that send information into the cerebellum. Further, just as there is a precise ordering in the way that information is sent from the cerebral cortex into the pons, and from there to the cerebellum, the feedback from the dentate nucleus of the cerebellum to the cerebral cortex is also precisely arranged. Thus, there are circuits, or anatomic loops that link higher order areas of the brain with the cerebellum, in a bidirectional manner. (This anatomical work is summarized in the 1997 monograph, The Cerebellum and Cognition.)
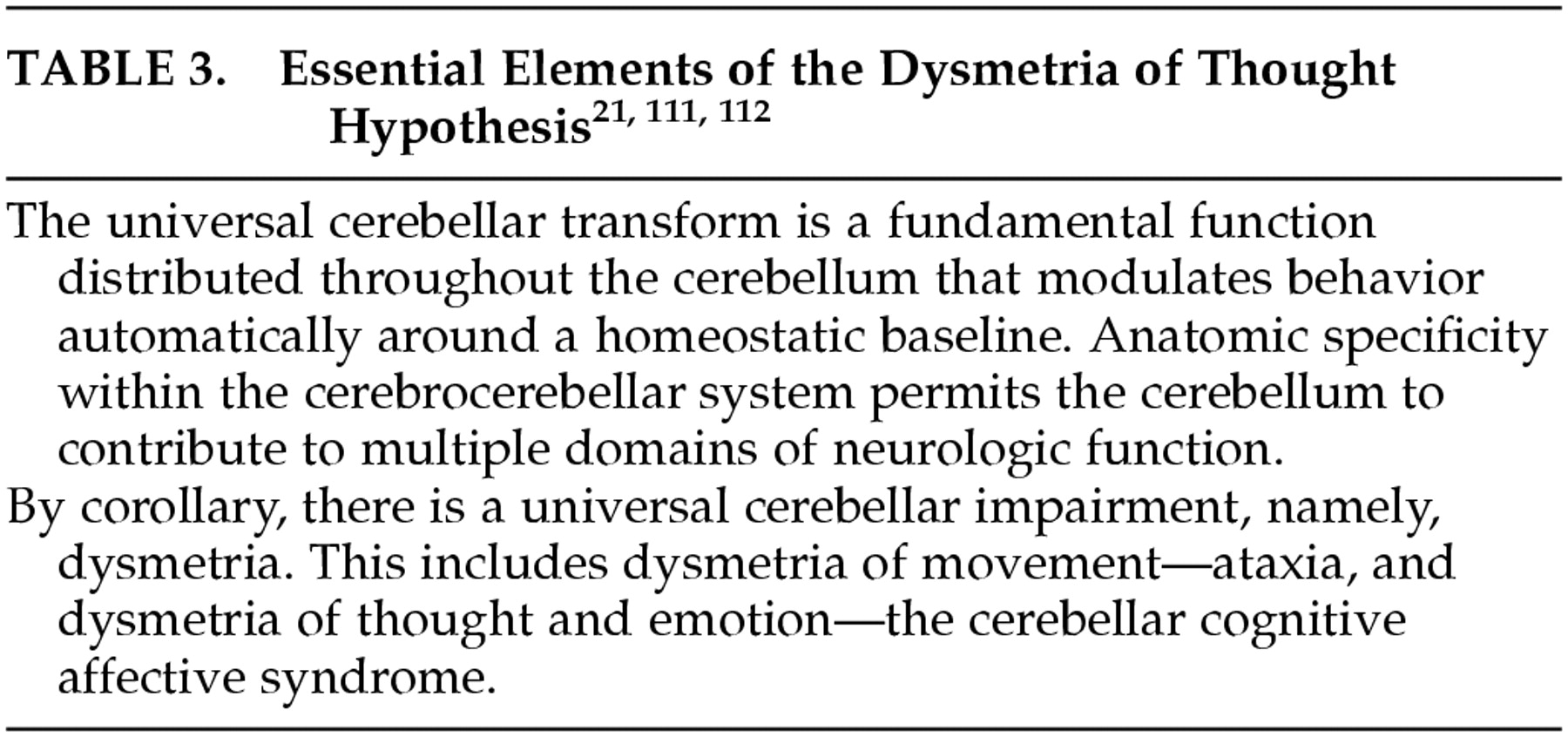
100It has become necessary to develop a new way of thinking about the cerebellum, one that takes all the various cerebellar roles into consideration. If cerebellum is not only a motor control device, then what does it do, and how does it do it? The early notion that the role of the cerebellum is to modulate neurologic function is compelling, and we have adopted and amended this as part of a conceptual approach to cerebellar function that I have referred to as the “dysmetria of thought hypothesis.”
21,111,112 In this view, because cerebellar anatomy is essentially uniform throughout the structure, the basic work that cerebellum does in the nervous system should be constant as well. This we have referred to as the universal cerebellar transform, characterized as the cerebellar modulation of behavior, serving as an oscillation dampener maintaining function automatically around a homeostatic baseline and smoothing out performance in all domains (
Table 3).
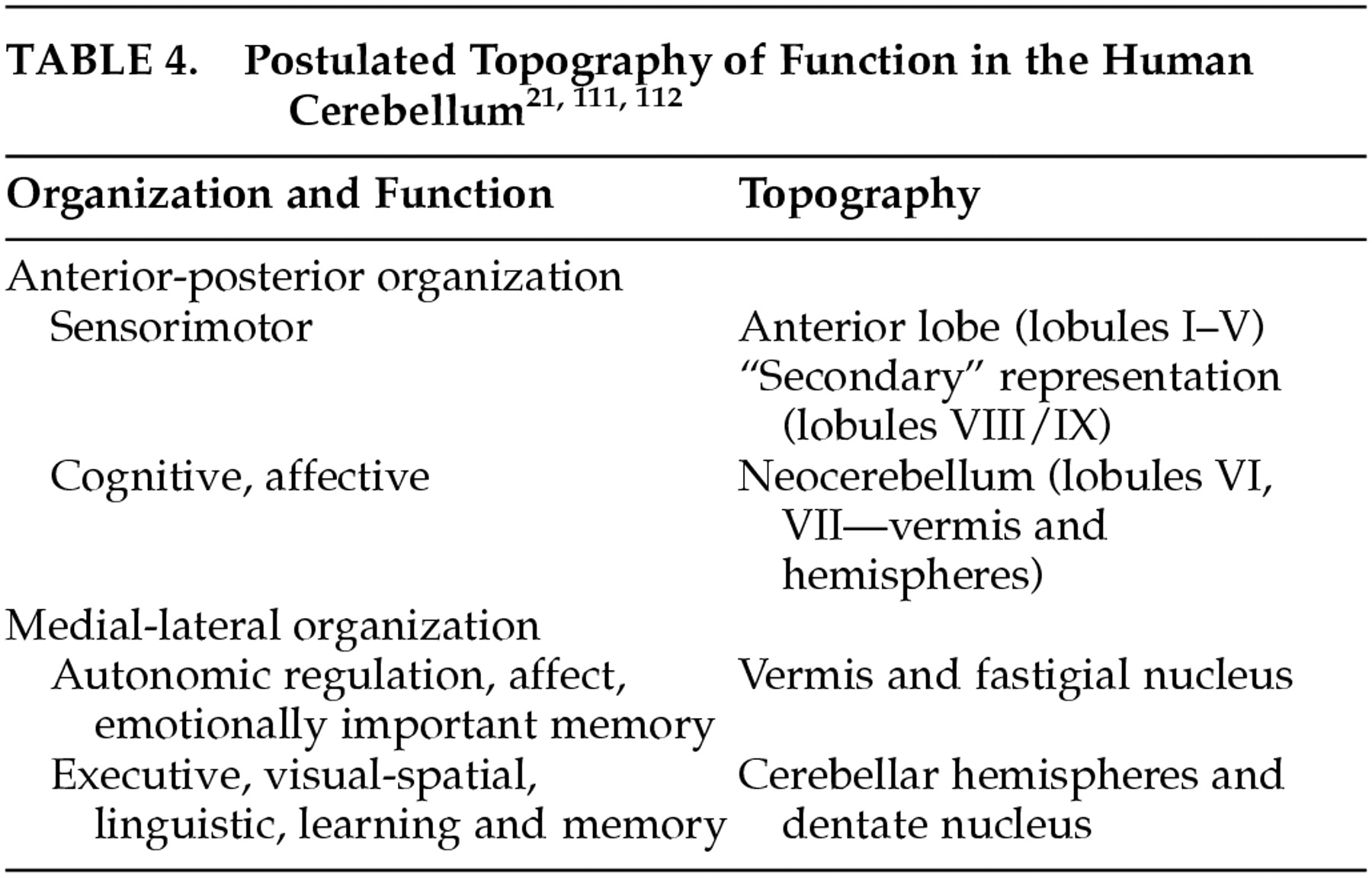
The specificity of the anatomic subcircuits in the cerebrocerebellar system indicates that different areas in the cerebellum interact with precise and different areas of the cerebral cortex. These anatomic subcircuits are the structural basis for putative functional subunits, and facilitate what appears to be topographic organization of motor and cognitive function in the cerebellum. In this proposed schema, the anterior lobe is mainly involved with motor control, whereas the posterior lobe is more concerned with higher order behaviors. Further, whereas the lateral parts of the posterior lobe are thought to be involved in cognitive operations, the vermis is considered to be the equivalent of the limbic cerebellum (
Table 4).
According to this hypothesis, the universal cerebellar transform is the essential functional contribution that the cerebellum makes to the distributed neural system. By corollary, therefore, there should be a universal cerebellar impairment. This universal cerebellar impairment, the hypothesis holds, is dysmetria. When the dysmetria involves the motor domain the various manifestations of ataxia are evident in extremity movements, eye movements, speech and equilibrium. However, when the dysmetria involves nonmotor functions subserved by cerebellum, this results in dysmetria of thought, or cognitive dysmetria, and manifests as the various components of the cerebellar cognitive affective syndrome.