Childhood or adolescent onset is reported by approximately one third to one-half of adults with obsessive-compulsive disorder (OCD).
1 Presently, it is unclear whether early and late onset OCD are different subtypes of the disorder or are part of a developmental continuum. Differences related to age of onset have been observed for sex distribution, family history of psychiatric illness, symptom subtypes, treatment response, and prevalence of comorbid psychiatric disorders.
2–5 Converging lines of evidence have implicated abnormality of frontostriatal circuitry in the etiology of OCD. Functional neuroimaging studies have generally reported hyperactivation of the orbitofrontal cortex, cingulate gyrus, and caudate nucleus.
6 Structural neuroimaging studies have been less consistent, with some investigations reporting abnormal volumes of the caudate nucleus and orbitofrontal cortex relative to healthy comparison subjects.
7–9 Neuropsychological studies have shown abnormalities in several cognitive domains in children and adults with OCD, including executive functions, memory, and visuospatial skills, albeit inconsistently.
10–12 The relationship between age of onset and structural and functional brain integrity has received scant empirical investigation. Patients with early and late onset OCD have been reported to show different patterns of motor control abnormalities during handwriting,
13 but did not differ on tests of verbal and visual memory.
14 In a single photon emission tomography (SPECT) study, patients with early onset OCD showed abnormal resting blood flow in the anterior cingulate gyrus, orbitofrontal cortex and cerebellum, while patients with late onset showed abnormalities in orbitofrontal cortex and precuneus, relative to healthy comparison subjects.
15 Another SPECT study failed to find any correlation between age of onset and resting blood flow.
16 In the present study, we extended prior research by evaluating patients with early and late onset OCD on several domains of neuropsychological functioning. Late onset has been defined from 13 years of age
15 to 18 years of age
14 and older. We chose to define late onset OCD as having onset of symptoms at 13 years of age or older, consistent with a SPECT study reporting significant differences between early and late onset OCD using this cutoff,
15 as well as to separate childhood onset from that occurring in adolescence or adulthood. Potential interactions between sex and age of onset on neuropsychological functioning were also evaluated, given prior evidence of a differential relationship between sex and age of onset in the disorder.
2,17 RESULTS
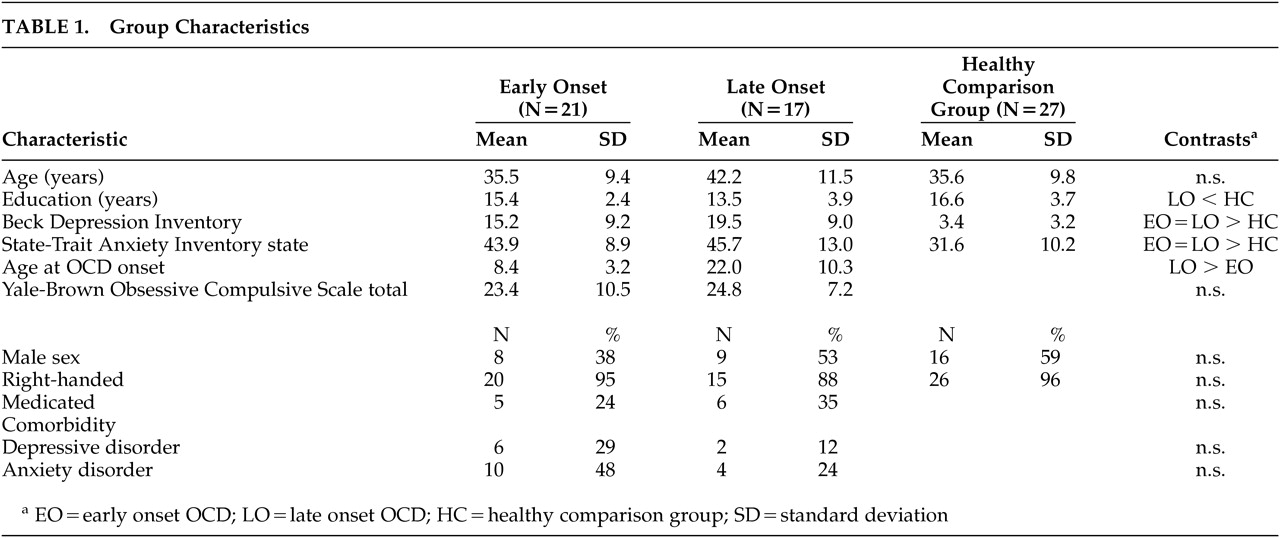
Demographic and clinical characteristics of the patient and comparison groups are presented in
Table 1. Group differences for age (F=2.65, df=2, 62, p=0.08), sex composition (χ
2=2.16, df=2, p=0.34) and handedness (χ
2=1.28, df=2, p=0.53) were not significant. However, the late onset group completed significantly fewer years of education than the comparison subjects (F=4.30, df=2, 62, p=0.02). The patient groups were also significantly more depressed (F=29.91, df=2, 62, p=0.001) and anxious (F=12.32, df=2,62, p=0.001) than the comparison subjects. The early and late onset patient groups did not differ with respect to symptom severity (F=0.41, df=1, 36, p=0.97) on the YBOCS, percentage of patients with comorbid depressive (χ
2=1.59, df=1, p=0.26) or anxiety disorders (χ
2=2.34, df=1, p=0.18), or percentage of patients receiving psychotropic medication (χ
2=0.49, df=1, p=0.49).
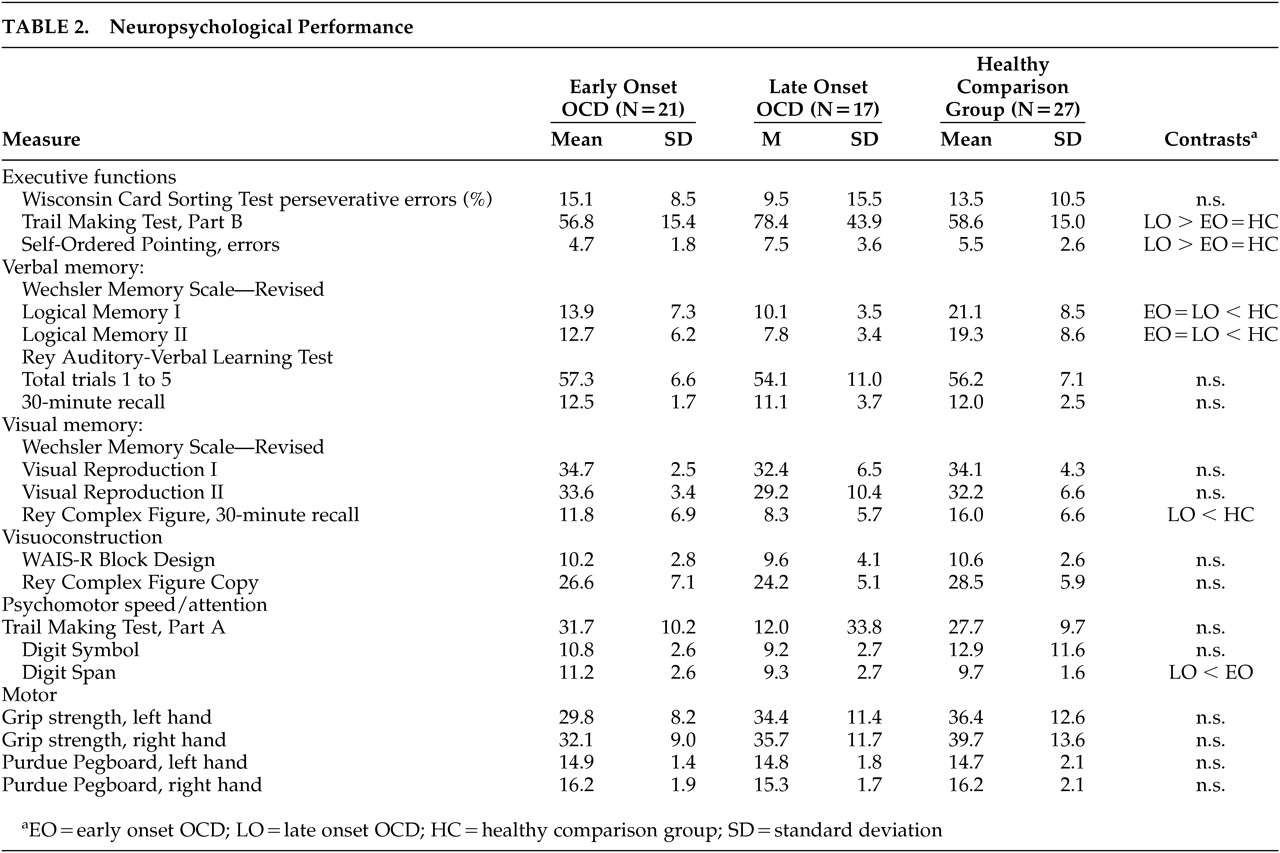
Descriptive statistics for neuropsychological test variables are presented in
Table 2. Results revealed that both patient groups recalled significantly less information on LM-I (F=14.09, df=2,62, p=0.001) and LM-II (F=15.53, df=2,62, p=0.001) than the comparison group. The late onset group also took longer to complete TMT-B (F=4.00, df=2,62, p=0.02) and made more errors on the Self-Ordered Pointing Test (F=5.33, df=2,62, p=0.007) than both the early onset and comparison groups, showed poorer delayed recall of the Rey Complex Figure (F=7.63, df=2,62, p=0.001), and had a lower digit span (F=4.04, df=2,62, p=0.02) than the early onset group. All group differences remained significant when controlling for education, depression or anxiety using analysis of covariance (p<0.05). No significant group × sex interaction was observed for any of the neuropsychological variables (p>0.05).
DISCUSSION
Results of the present study indicate that patients with early and OCD differ in their pattern of neuropsychological functioning. Patients with early onset OCD only showed poorer memory for prose passages relative to healthy comparison subjects. In contrast, patients with late onset OCD showed poorer performance on tests of verbal and visual memory, as well as executive functions and auditory attention. These findings support results of a recent SPECT study indicating differences in cerebral blood flow related to age of onset in OCD.
15 The observation of impaired executive functions in late onset OCD is consistent with a hypothesized “primary” subtype of the disorder that is characterized by late onset and frontal systems dysfunction.
2 Executive dysfunction has been observed in several studies of OCD patients that were not classified based on age of onset.
12,31 However, a number of recent studies have found that neuropsychological differences between patients with OCD and healthy comparison subjects, particularly executive dysfunction, are accounted for by depression.
32,33 In the present study, the patient groups did not differ with respect to self-reported mood or comorbid depressive or anxiety disorders. Furthermore, neuropsychological differences between the patient and comparison groups could not be accounted for by self-reported depression or anxiety. Thus, executive dysfunction in late onset OCD cannot be accounted for by co-occurring mood symptoms.
A neurodevelopmental subtype of OCD has also been proposed that is characterized by childhood onset, predominantly male sex, poorer response to treatment, and neuromotor abnormalities.
34 While our sample sizes were modest, results indicate that sex does not moderate the relationship between age of onset and neuropsychological functioning. The lack of group differences for motor skills appears inconsistent with evidence of greater prevalence of tic disorders in early onset OCD
35 and results of a recent study of handwriting in adults with OCD that found earlier onset to be associated with disturbed movement sequencing, while later age of onset is related to greater difficulty performing movements requiring more complex motor control.
13 However, we employed relatively simple tasks involving handgrip strength and motor speed, neither of which required sequencing or complex movements. Further studies of early and late onset OCD employing motor tasks with sequencing and increased movement complexity requirements would likely prove informative. In addition, information regarding the presence of tic disorder in our sample was unavailable. Assessment of motor control in relation to age of onset and presence of tic disorder would be helpful to further evaluate the validity of a neurodevelopmental subtype of OCD.
Discrepancies between studies investigating correlates of age of onset in OCD may be due in part to the use of different cutoffs to define early and late onset groups. We defined late onset as that occurring at age 13 or later, based on results of a recent SPECT study
15 and in order to separate childhood onset from that in adolescence or adulthood. While we believe that this cutoff is reasonable, based on the above arguments, we acknowledge that both our cutoff and that used by other authors is somewhat arbitrary. Studies with large samples of patients with OCD would permit the use of multivariate statistical techniques to identify significant peaks in onset of symptoms across the life span, thus allowing for a more empirically based classification. Furthermore, while a number of studies of adult patients have employed retrospective self-report of OCD symptom onset,
14,36 our study must be interpreted in the context of the limitations inherent in this methodology.
We did not observe any difference in memory functioning between patients with early and late onset OCD, consistent with the only other published study to evaluate the relationship between age of onset and neuropsychological functioning.
14 However, our late onset group showed poorer performance on a visual memory test, and both patient groups performed worse on a verbal memory test, relative to the comparison group. It is unknown whether this visual memory impairment in our late onset group would also have been observed by Henin et al. if they had included a healthy comparison group,
14 or if they had used our cutoff of 12 years of age and younger rather their use of under 18 years of age for defining early onset OCD.
In summary, the present findings of neuropsychological differences between early and late onset OCD raise the possibility of differential neurobiological substrates related to age of onset. Discrepancies between prior neuropsychological studies of OCD may be due in part to inclusion of patients with widely varying ages of onset. Further studies examining neuropsychological functioning in early and late onset OCD in larger samples are warranted. The present findings support recent SPECT evidence of differential patterns of brain activation in early and late onset OCD.
15 However, the precise neural circuitry underlying the patterns of cognitive functioning observed in the present investigation remains unclear and does not readily map onto the observed regional differences in the aforementioned SPECT study. Positron emission tomography and functional MRI during the performance of cognitive tasks would be helpful in directly evaluating the relationship between cognitive functions and underlying neural circuitry in early and late onset OCD.