It has been postulated that experiencing joy and pleasure depends on dopaminergic reward mechanisms in the limbic system, which are thought to be the basis of motivation, drive, and activation.
2 In Parkinson's disease, degeneration of dopaminergic neurons involves motor structures, including basal ganglia, but also structures of the limbic system.
3 Degenerative processes in Parkinson's disease may affect dopaminergic reward mechanisms and lead to anhedonia, loss of motivation, avolition and apathy.
4 These pathophysiological mechanisms could explain effects of pramipexole, a novel dopamine agonist, on anhedonia and depression found in animal experiments
5,6 and patients with major depressive disorder.
Anhedonia, a core symptom of depression,
7 correlates with motor retardation in patients with major depressive disorder.
8 Anhedonia has been assumed to be a frequent symptom in depressed patients with Parkinson's disease.
9 Like in major depressive disorder, anhedonia may also have an impact on motor functioning and activities of daily living in Parkinson's disease. However, to our knowledge, no data exist regarding frequency and relevance of anhedonia in Parkinson's disease.
Therefore, the aim of this open study was to investigate depressive symptoms in Parkinson's disease and test the hypotheses that anhedonia is more frequent in patients with Parkinson's disease compared with healthy comparison subjects, that anhedonic patients with Parkinson's disease show more severe parkinsonian symptoms compared to nonanhedonic patients and that anhedonia is reduced during treatment with pramipexole.
METHODS
In this prospective, observational, open study, we included patients (N=657) with clinical diagnosis of Parkinson's disease (presence of 2 of 3 cardinal features [tremor, bradykinesia, rigidity] and
L-dopa responsiveness) at 298 study sites (in- and outpatients) if pramipexole as an add-on to L-dopa was clinically indicated (T1). Dosage was adjusted depending on efficacy and tolerability in weekly intervals allowing a maximal dose of 3×1.5 mg/day. Neurological and psychiatric examinations were performed at baseline (T1) and at the end of a maintenance period of 9 weeks on an average (T2). Exclusion criteria were psychotic symptoms (delusions, hallucinations, etc.), moderate to severe dementia and contraindications for pramipexole treatment including hypersensitivity to the drug. Healthy volunteers (N=50) were recruited for comparison from employees of the University of Kiel and their relatives. Comparison subjects were excluded if they had any psychiatric or neurological disease at present or had a history of such a disease which was assessed by a standardized interview (MINI)
10 and motor scales (ESE)
11 and clinical examination.
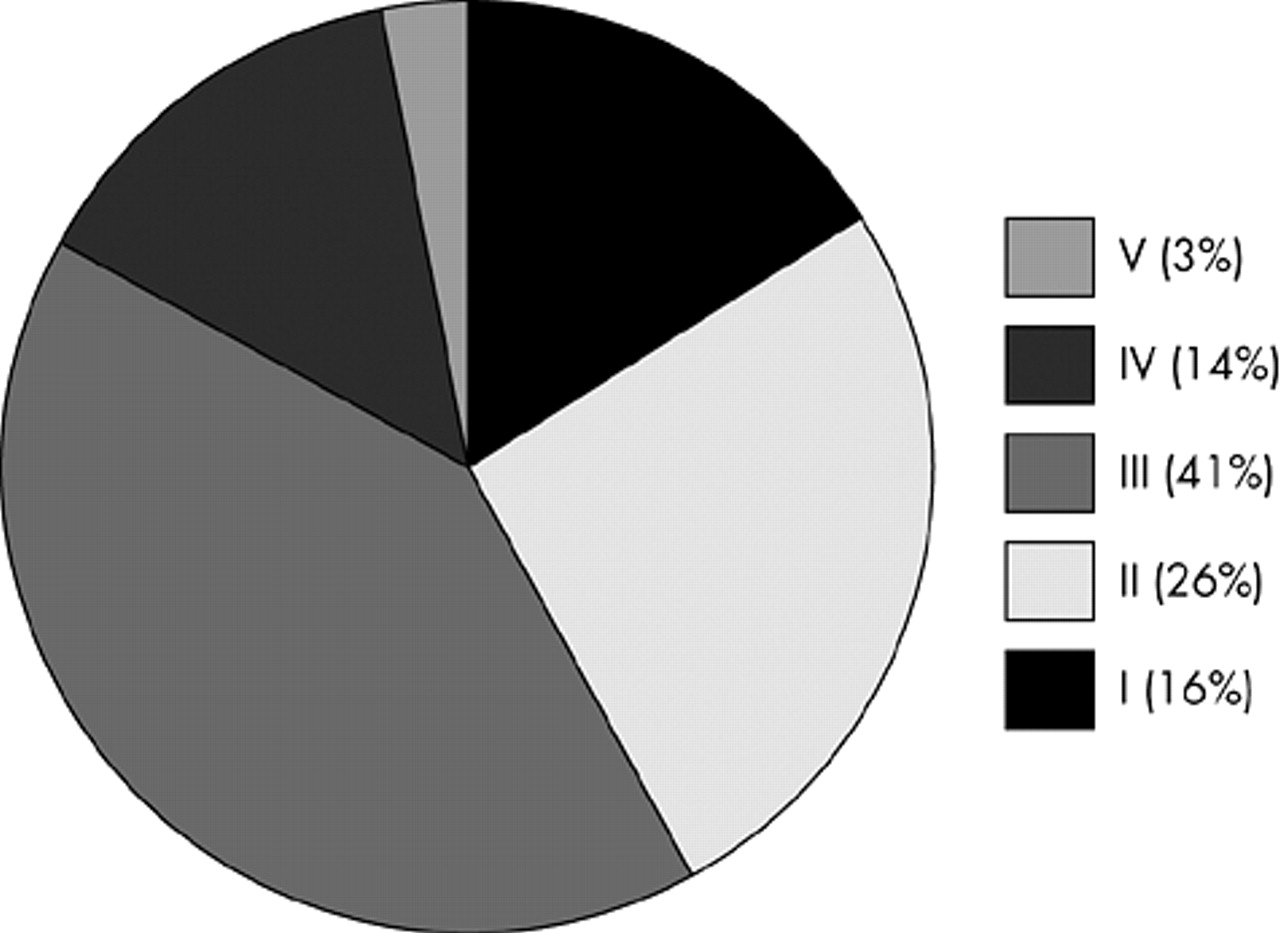
For staging of the disease, we applied the Hoehn and Yahr Scale (ranging from 0 to 5; stage 1 = unilateral symptoms, stage 5 = restricted to wheelchair or bed if unsupported by others).
12 Trained observers rated severity of motor and nonmotor symptoms of Parkinson's disease using the Short Parkinson's Evaluation Scale (SPES) (range=0 to 98, higher values representing more severe symptoms) and SPES subscales motor functioning, psychopathogy, depression, and activities of daily living.
13 Subjects were categorized as depressed on the basis of SPES depression scores (0= no, 1= mild, 2= moderate, 3= severe depressive symptoms). Anhedonia was assessed by the German version of the self-rating Snaith-Hamilton Pleasure Scale (SHAPS-D) (range=0–14, higher values representing more severe anhedonia; cutoff score for anhedonia≥3).
14,15Statistical analysis included descriptive statistics and testing for normal distribution. We analyzed group differences using chi-square tests and unpaired and paired t tests (two-tailed). The upper limit for significance to reject H0 was p<0.05. We analyzed correlations using Spearman correlation coefficient (rs) (two-tailed, pairwise exclusion of missing data) where the upper level of significance to reject H0: rs = 1 was set at p<0.05. In order to clarify the factor structure of the SHAPS-D in patients with Parkinson's disease, we performed a principal component analysis of the 14 items and evaluated the reliability of SHAPS-D with Cronbach alpha as measure of the internal consistency.
RESULTS
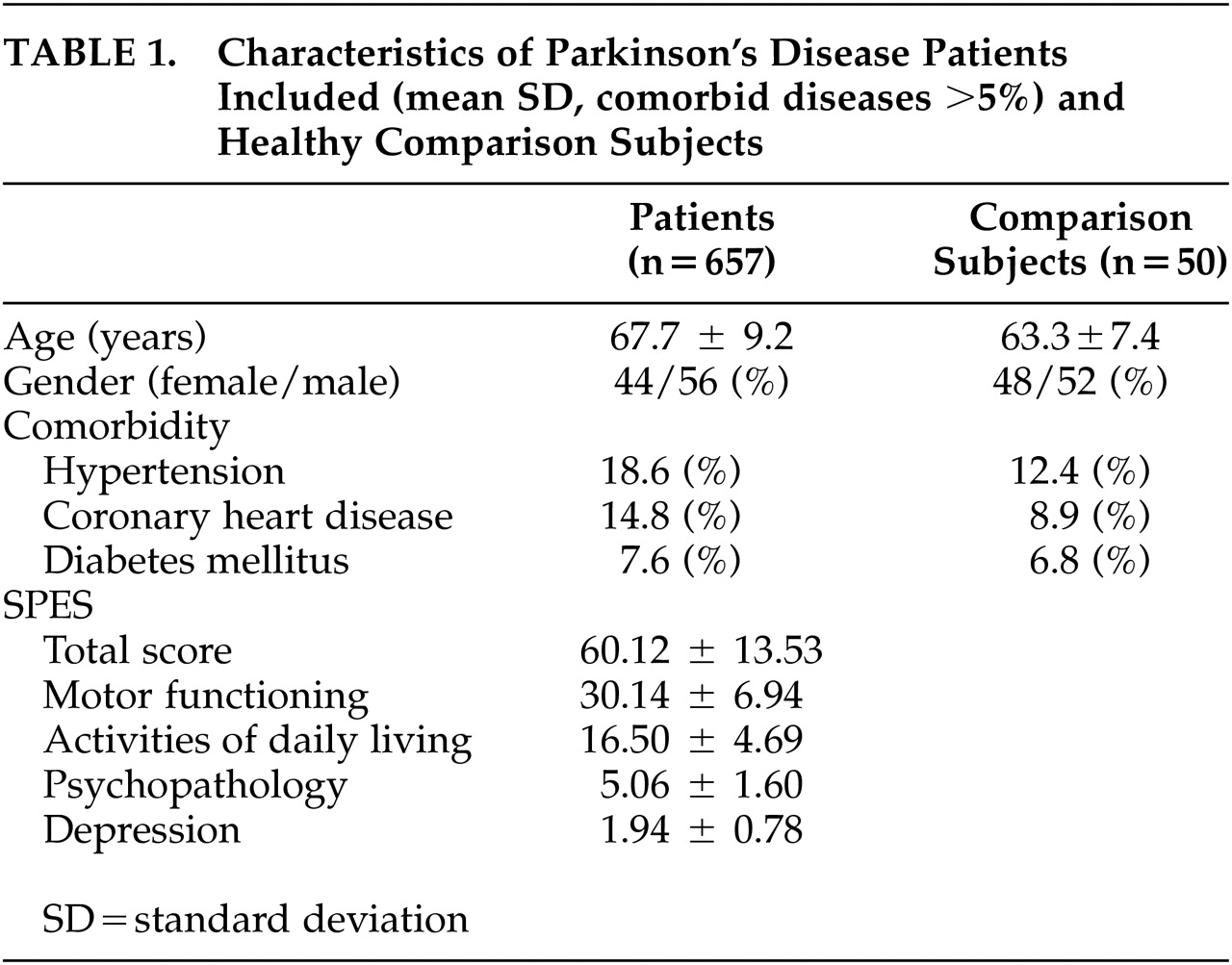
Each study site recruited 2.2 patients on average (SD 3.2, range=1–40). The characteristics of all patients included are given in
Table 1 and
Figure 1. Most patients (86%) received comedication in addition to antiparkinson therapy. In addition, patients were treated with tri- and/or tetracyclic antidepressants (7.1%), hypnotics (3.6%), selective serotonin and/or norepinephrine reuptake inhibitors (3.4%), St. John's Wort (2.2%), MAO-inhibitors (0.3%), and neuroleptics (0.3%).
All patients with Parkinson's disease (95.28% [N=626]) included in the study (N=657) completed the SHAPS-D Questionnaire. Cronbach alpha, a test for internal consistency, was 0.92 in nondepressed Parkinson's disease patients and 0.90 in depressed Parkinson's disease patients. Factor analysis (principal component analysis) showed significant loading of all items of the SHAPS-D on one factor which explained 75% of the total variance.
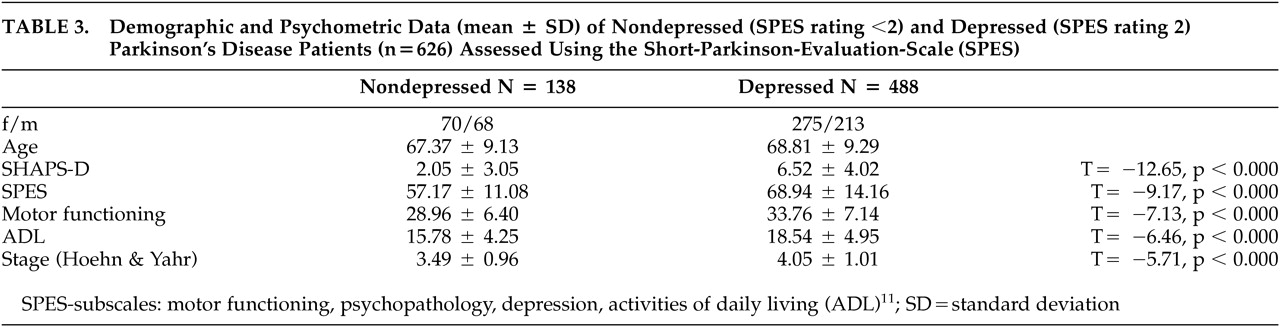
Snaith-Hamiltion Pleasure Scale-D scores (1.62 ± 1.57) of healthy volunteers (N=50; age 63.3 ± 7.4 years, 48% female) differed significantly from scores (3.39 ± 3.68, p<0.001) of patients with Parkinson's disease (N=626, age 67.7 ± 9.2 years). Analyzing depressed and nondepressed Parkinson's disease patients separately, we did not find a significant difference in SHAPS-D scores between healthy comparison subjects and nondepressed patients with Parkinson's disease (N=488; 2.51 ± 4.02, p>0.05), but significantly higher scores in depressed patients with Parkinson's disease (N=138; 6.52 ± 4.02, p<0.0001). Anhedonia was present in 79.7% of Parkinson's disease patients with depression.
We did not find a significant correlation between age and SHAPS-D scores either in the whole sample (N=626; r=0.071, p=0.07) or in depressed (N=138; r=0.136, p=0.1107) or nondepressed (N=488; r=0.015, p=0.7446) patients with Parkinson's disease. Patients in earlier stages of the disease (Hoehn and Yahr <=2) had anhedonia less often, compared with patients in more advanced (>2) stages (chi-square 20.81, df=4, p<0.001).
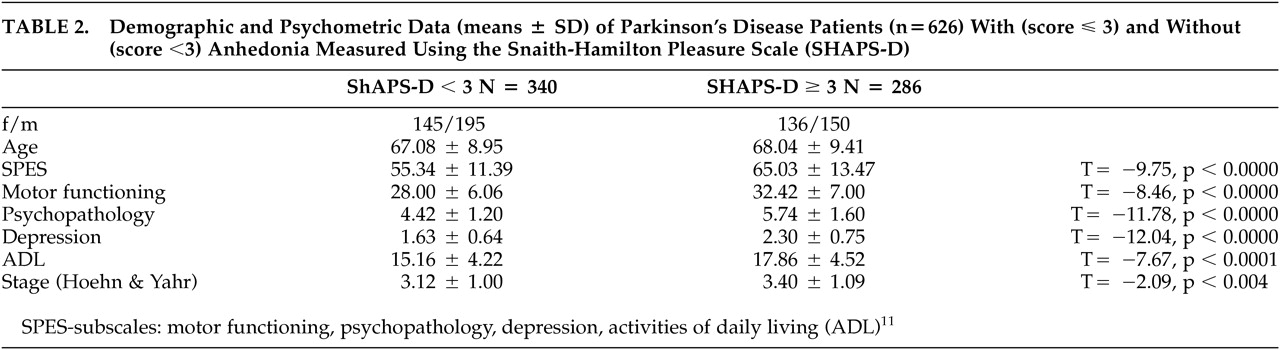
We divided the total sample (N=626) using the previously defined
12,13 cutoff score of ≥3 into an anhedonic subgroup (N=286; 45.7%, score≥3) and a nonanhedonic subgroup (N=340; 54.3%, score<3).
Table 2 shows characteristics of the two subgroups and differences between them.
On the basis of SPES depression scores (0=no, 1=mild, 2=moderate, 3=severe depressive symtoms), 31% of the patients showed no depression, 47% mild, and 22% moderate to severe depression. We dichotomised the study population (N=626) into a moderately to severely depressed subgroup (score≥2, N=138, 22%) and a non- to mildly depressed subgroup (score<2, N=488, 78%).
Table 3 gives characteristics of the two subgroups and differences between them. If patients with mild depressive symptoms were included (score≥1), we allocated 69% to the depressed and 31% to the nondepressed subgroup.
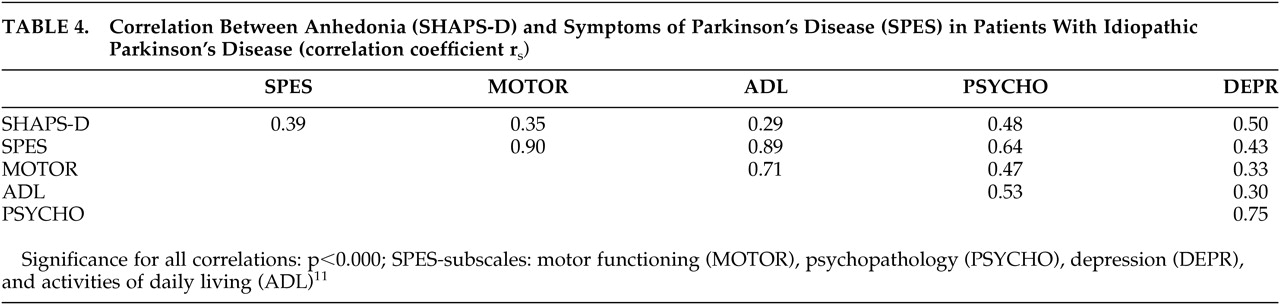
The relationship between anhedonia (SHAPS-D) and symptoms of Parkinson's disease (SPES) is presented as a correlation matrix in
Table 4. Significance of correlation did not change when we calculated partial correlation for age as the control variable. We calculated partial correlation between various variables and SHAPS-D for depression and motor functioning as control variables. When we controlled for depression, correlation between SHAPS-D and SPES total (r
s= 0.23, p>0.05), activities of daily living (ADL) (r
s= 0.08, p>0.05), and SPES motor functioning (r
s= 0.16, p>0.05) was not statistically significant. When we controlled for motor functioning, correlation was not significant between SHAPS-D and SPES total (r
s= 0.11, p>0.05), ADL (r
s= 0.07, p>0.05), but significant between SHAPS-D and depression (r
s= 0.49, p>0.000).
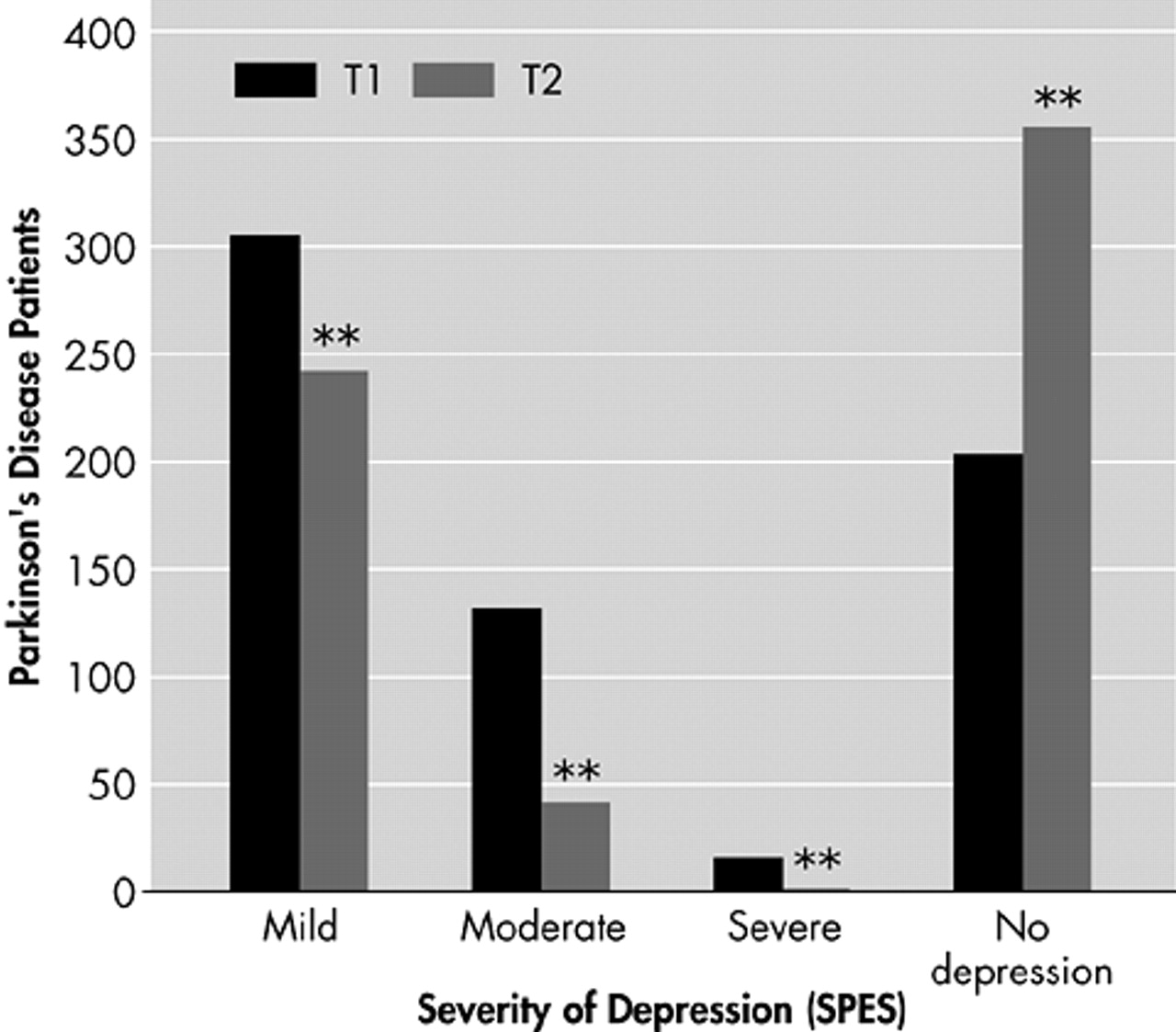
Dosage was adjusted depending on efficacy and tolerability in weekly intervals allowing a maximal dose of 3×1.5 mg/day. At T2, the average dose of pramipexole was 1.0 ± 0.6 mg/day (range=0.3 to 4.2). Anhedonia was present in N=286 (45.7%) patients at T1 and N=160 (25.5%) at T2. In depressed PD patients (N=138), frequency of anhedonia significantly decreased from 74.3% to 45.3% (χ2 = 34.30, df=1, p<0.001), and in nondepressed Parkinson's patients (N=488) from 34.6% to 18.3% (χ2 = 60.377, df=1, p<0.001), respectively. Frequency of depression at T1 and T2 is depicted in
Figure 2.