Tardive dyskinesia (TD) and extrapyramidal side effects are frequent side effects of treatment with first-generation antipsychotics (FGAs). Different hypotheses have been considered in determining the causes of TD, a disabling adverse iatrogenic effect of antipsychotics. One possible pathophysiological explanation is neuronal cell damage from free radicals induced by antipsychotics.
1 This hypothesis is supported by evidence of elevated levels of lipid peroxidation products and decreased vitamin E levels in dyskinetic patients.
2,3 In animal studies, oxidative stress and elevated levels of lipid peroxidation have been implicated in haloperidol toxicity.
4 Evidence to support these animal studies includes elevated levels of lipid peroxidation with FGA treatment in psychotic patients.
5 The clinical effects of vitamin E on TD are only positive in the animal model but not in patients with TD itself.
6 However, the work of an Israeli group showed a significant improvement of TD in 22 patients with schizophrenia, with the antioxidant melatonin in a double-blind, placebo-controlled, crossover study.
7,8 Oxidative stress has been assumed to be a pathogenetic mechanism associated with neurodegeneration.
9 The peroxidation of membrane lipids is associated with a wide variety of toxicological effects. Lipid peroxidation itself reflects the interaction between molecular oxygen and polyunsaturated fatty acids, leading to oxidative deterioration of the latter with the production of several breakdown products. An established marker of oxidative stress is malondialdehyde (MDA), which can easily be detected.
10,11,12 As patients with FGAs more often have extrapyramidal symptoms (EPS) and TD, our hypothesis is that FGAs might lead to higher lipid peroxidation, which might induce EPS, and with long-term treatment, TD. Active peroxidation can be recognized by increased levels of lipid peroxidation in plasma.
2,13,14RESULTS
Demographic Characteristics
Patients with schizophrenic disorders (N=76), schizoaffective disorders (N=5) and mood disorders (N=11) were included in the study. The mean age and standard deviation (SD) of the FGA group was 38.5 years (SD=10.8) (nine women and 12 men; seven nonsmokers and 14 smokers). The second-generation antipsychotic (SGA) group incorporated 70 patients, aged 34.8 years (SD=12.1), 30 of whom were women and 40 of whom were men. Age within the subgroups of the SGAs were as follows: amisulpride (AMI) mean=37.8 (SD=7.6); risperidone (RIS) mean=35.0 (SD=16.4); clozapine (CLO) mean=36.3 (SD=14.4); olanzapine (OLA) mean=34.8 (SD=11.1). No significant statistical differences were found between age in the different SGA groups or between SGAs and FGAs. Smokers in the SGA group were dominant, with 49 patients. Twenty one patients were nonsmokers.
Treatment Groups
The FGA group consisted of patients treated with haloperidol (N=5) or flupentixol (N=17). Mean haloperidol dosage was 6.2 mg/day, and mean flupentixol dosage was 3.5 mg/day. In the SGA group, the 70 patients were divided in subgroups, with AMI (N=6; mean=447.2 mg/day); RIS (N=17; mean=4.7 mg/day); CLO (N= 12; mean=337.0 mg/day); OLA (N=22; mean=13.9 mg/day); and QUE (N=13; mean=363.4 mg/day).
Psychopathology
There was no difference concerning the BPRS scores on day 0 (FGAs mean=47.0, SD=13.3, versus SGAs mean=42.5, SD=9.7); day 7 (FGAs mean=42.1, SD=9.7, versus SGAs mean=39.7, SD=8.7); and day 21 (FGAs mean=38.8, SD=10.3, versus SGAs mean=35.4, SD=8.9) between both groups (Mann-Whitney tests: p=0.073 to 0.241, U 553 to 619, respectively).
Ratings of Extrapyramidal Symptoms
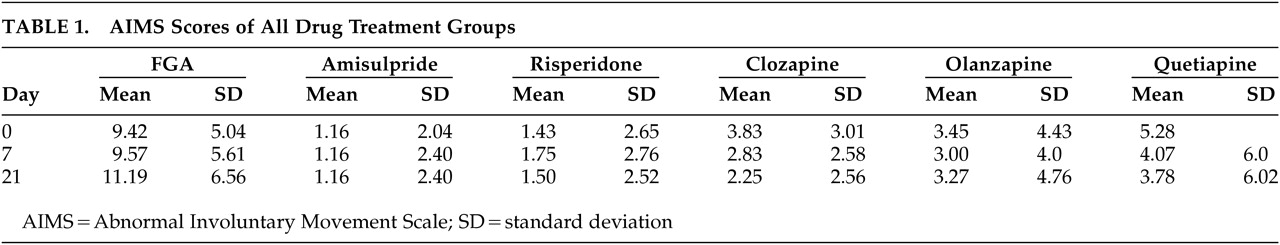
Mean AIMS scores on day 0, day 7, and day 21 differed significantly between FGAs and SGAs (each p<0.0001). The highest AIMS scores were found in the FGAs, which also increased during the 3-week study. In the AMI group, there was no change of EPS during the study, and patients on RIS and OLA changed insignificantly. Patients with QUE and CLO had the most pronounced benefit on the AIMS during this study. The results for the different compounds are shown in
Table 1.
Laboratory Results
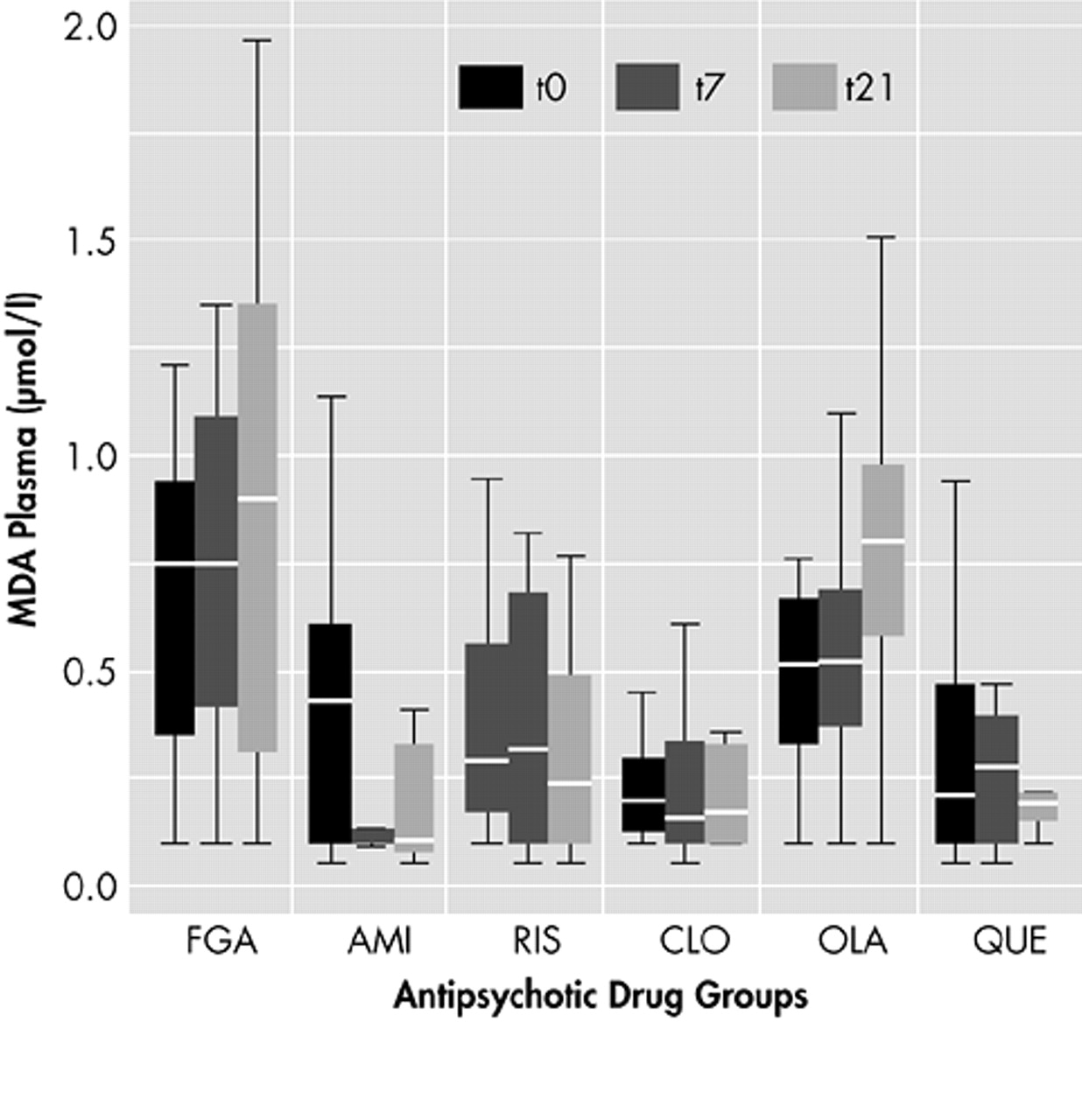
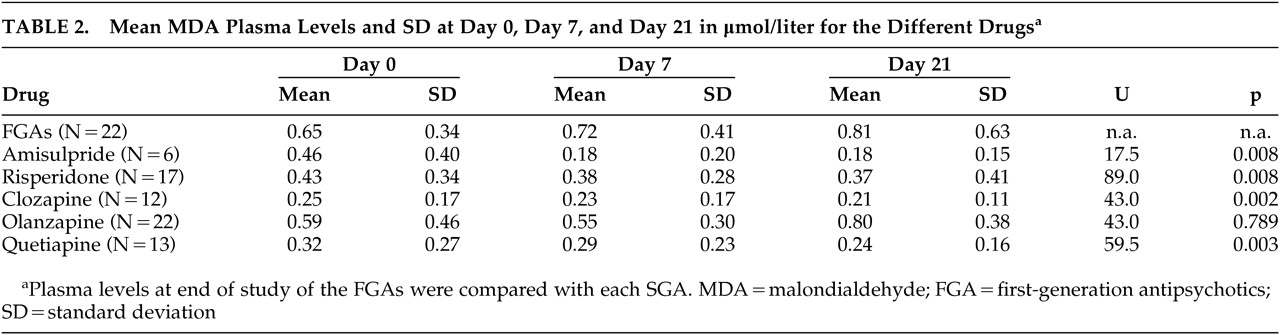
Most MDA levels were within normal range (<1.0 μmol/liter). No significant differences of MDA results were found between smokers and nonsmokers, between men and women, or among patients with concomitant medications. Other possible confounding factors such as duration of illness or age at onset had no influence on MDA levels (data not shown). At the end of the study on day 21, the highest MDA plasma levels were found in patients who received FGAs (mean 0.81). The lowest levels of MDA were localized in blood samples of patients with clozapine (mean 0.21) and amisulpride (mean 0.18). This was followed by the samples of quetiapine (mean 0.24) and risperidone (mean 0.37). Samples of patients with olanzapine had higher plasma levels of MDA within the SGAs (mean 0.80) and missed significant differences compared to FGAs. All other SGAs had significantly lower levels of MDA than patients with FGAs after 21 days. (For details see
Table 2 and
Figure 1.)
DISCUSSION
The most important finding of this study is that plasma levels of membrane lipid peroxidation products were found to be elevated significantly in patients treated with FGA, compared to most SGAs after a 3-week longitudinal study of antipsychotic treatment. It is well established that oxidative stress within the CNS is reflected in plasma.
13,14 There is also evidence that psychotic disorders might impair antioxidant defense and increase lipid peroxidation, as antipsychotic treatment itself increases oxidative stress and induces irreversible neuropathological changes in animal models.
21,22,23,24To our knowledge, this is the first comparative study of oxidative stress in a population of patients being treated with FGAs and SGAs. An animal study with a similar hypothesis but different markers was published, of which the main results substantiate our conclusions.
25 For most SGAs, we have shown significant differences to FGAs, which might explain the different incidence of EPS between FGAs and SGAs.
26,27 Currently, we cannot explain why patients on olanzapine had higher MDA levels than other SGAs, but patients on olanzapine were not more indisposed than other patients in this study, according to BPRS ratings.
Oxidative stress induced by antipsychotic treatment is a hypothesis that should be taken into account concerning EPS and TD.
2 There is also evidence of an impaired antioxidant defense and increased oxyradical-mediated cellular injury in patients with nonaffective psychoses who have never been treated with antipsychotics.
21 For a long time, the theory that striatal postsynaptic dopamine receptor supersensitivity causes TD has been widely accepted, but new evidence may change this model.
11 Recently, a new hypothesis combining the facts that antipsychotics enhance striatal glutamatergic neurotransmission by blocking presynaptic dopamine receptors and cause neuronal damage by oxidative stress was presented.
3 Using the model of glutamatergic neurotransmission and the evidence of long-lasting persistence of FGAs in human brain tissue and the “fast dissociation hypothesis” of antipsychotics as explanation for “atypicality” of SGAs,
3,28,29 our results may explain why SGAs, such as amisulpride, clozapine, and quetiapine, showed lower levels of oxidative stress in our study.
This study contains methodological limitations, however. One significant limitation is the open, nonblinded design, without a defined washout period. A washout-period for a longer time in acute patients would have been problematic from an ethical point of view. Further, the small sample size, the use of only one marker, and no measurement of the antioxidant defense system, as used by Parikh and co-workers, are limiting factors.
25 For future studies on lipid peroxidation during treatment with SGAs and FGAs, isoprostanes could be used in combination with MDA.
30,31The role of free radicals and lipid peroxidation in different strategies of antipsychotic treatment requires further research. Obviously, these findings from an open, comparative study require replication.
ACKNOWLEDGMENTS
This study was supported in part by AstraZeneca Pharmaceuticals, the Eli Lilly and Company, and Janssen-Cilag Pharmaceutica-Cilag.
This study was presented in part at the Collegium Internationale Neuro-Psychopharmacologicum (CINP), Montreal, 24-27 June 2002; and the Nordic Psychiatric Congress (NPC), Reykjavik, 13–16 August 2003.