A n extensive body of research provides converging evidence for an important role of sleep in learning, brain plasticity, and cognitive functions.
1 –
3 However, crucial questions still remain open—whether memory consolidation is linked to particular sleep stages and whether this interrelationship is altered in pathological mental conditions like schizophrenia.
In addition to its relationship to sleep homeostasis, delta wave activity is considered an important aspect of sleep in the link to cognitive performance. Bodizs et al.
4 found an association between temporo-lateral low delta (<1.25 Hz) activity during sleep and visual memory performance in epileptic patients. Anderson and Horne
5 described correlations between frontal low delta (<1 Hz) sleep EEG and neuropsychological tests specific to the prefrontal cortex. Further, a local increase in delta activity (1 to 4 Hz) during sleep after learning correlated with improved performance in an implicit learning paradigm the next morning.
6Schizophrenia is characterized by psychotic and deficit symptoms, such as cognitive impairments. We recently described a correlation of visuospatial memory impairments and a decrease in the amount of slow wave sleep (SWS) in schizophrenia.
7 Furthermore, in addition to disturbances, especially of non-REM (rapid eye movement) sleep,
8 a decrease in delta (0.5–4 Hz) activity during sleep has been reported.
9 Therefore, we performed delta power analyses in sleep in relation to neuropsychological tasks in healthy subjects and schizophrenia patients. We hypothesized that delta activity is associated with neuropsychological performance in healthy subjects and that this link might be impaired in schizophrenia. Because cognitive symptoms play a major role in quality of life and predict functional outcome in schizophrenia,
10 research in this field is essential and important.
DISCUSSION
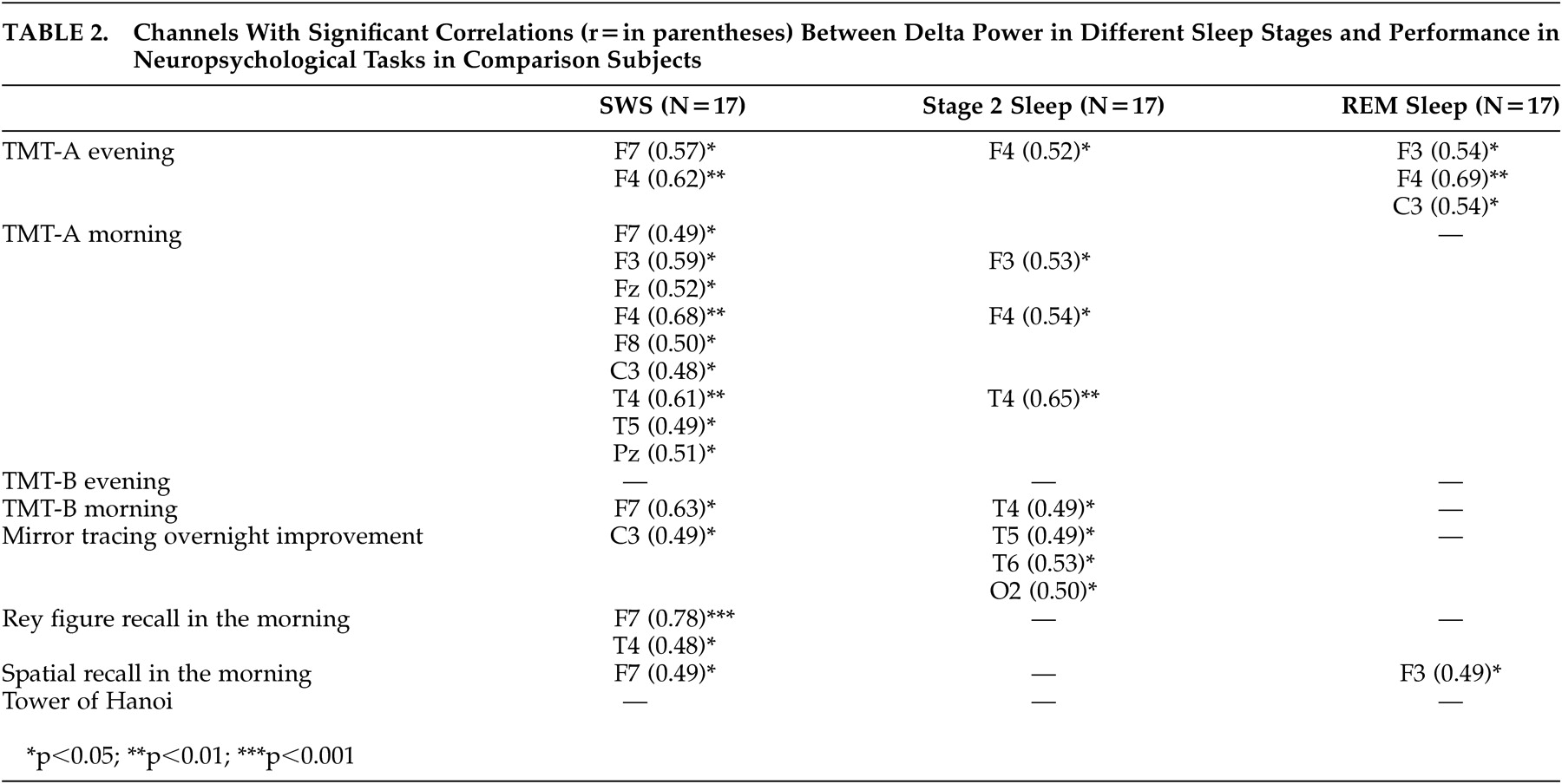
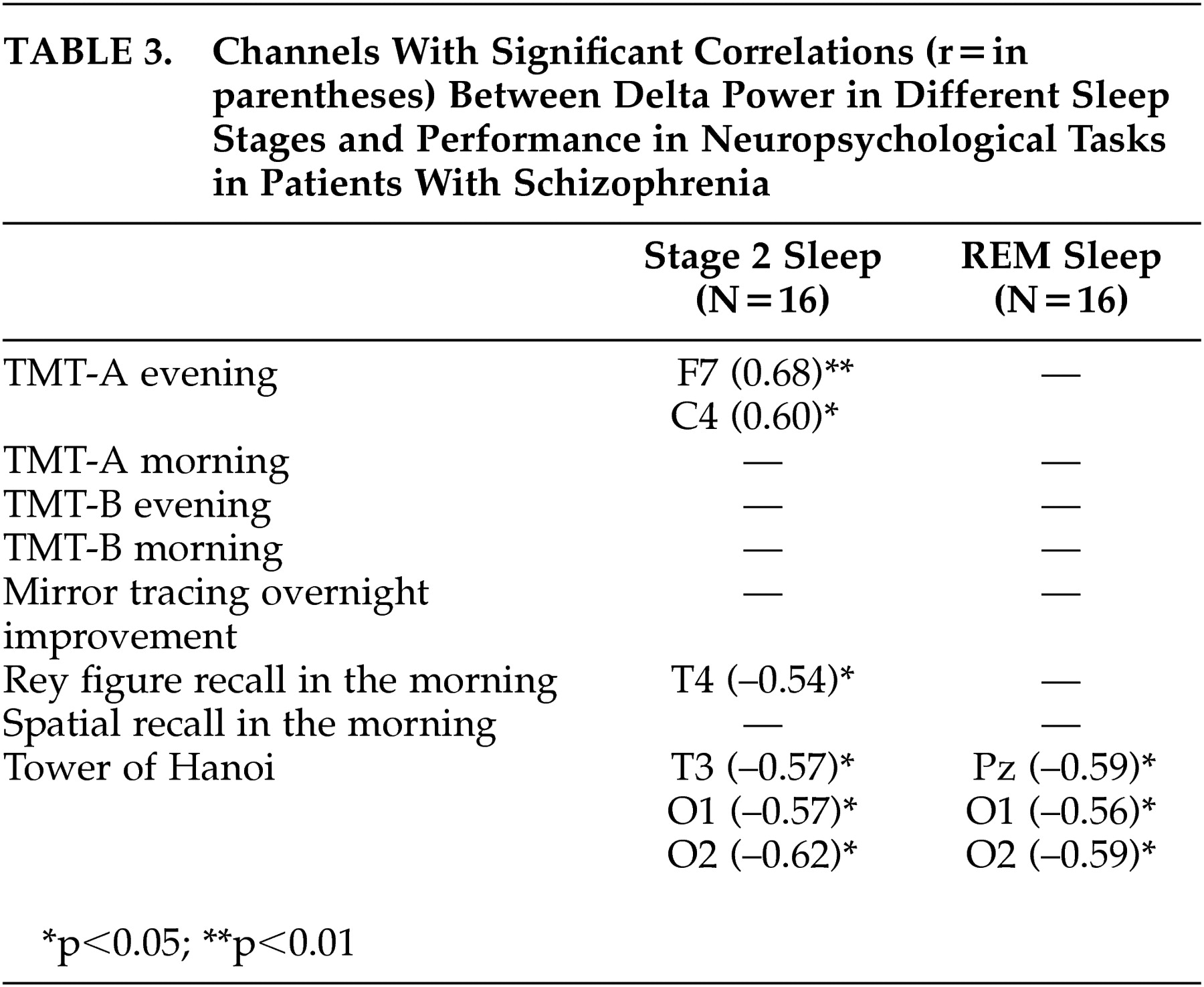
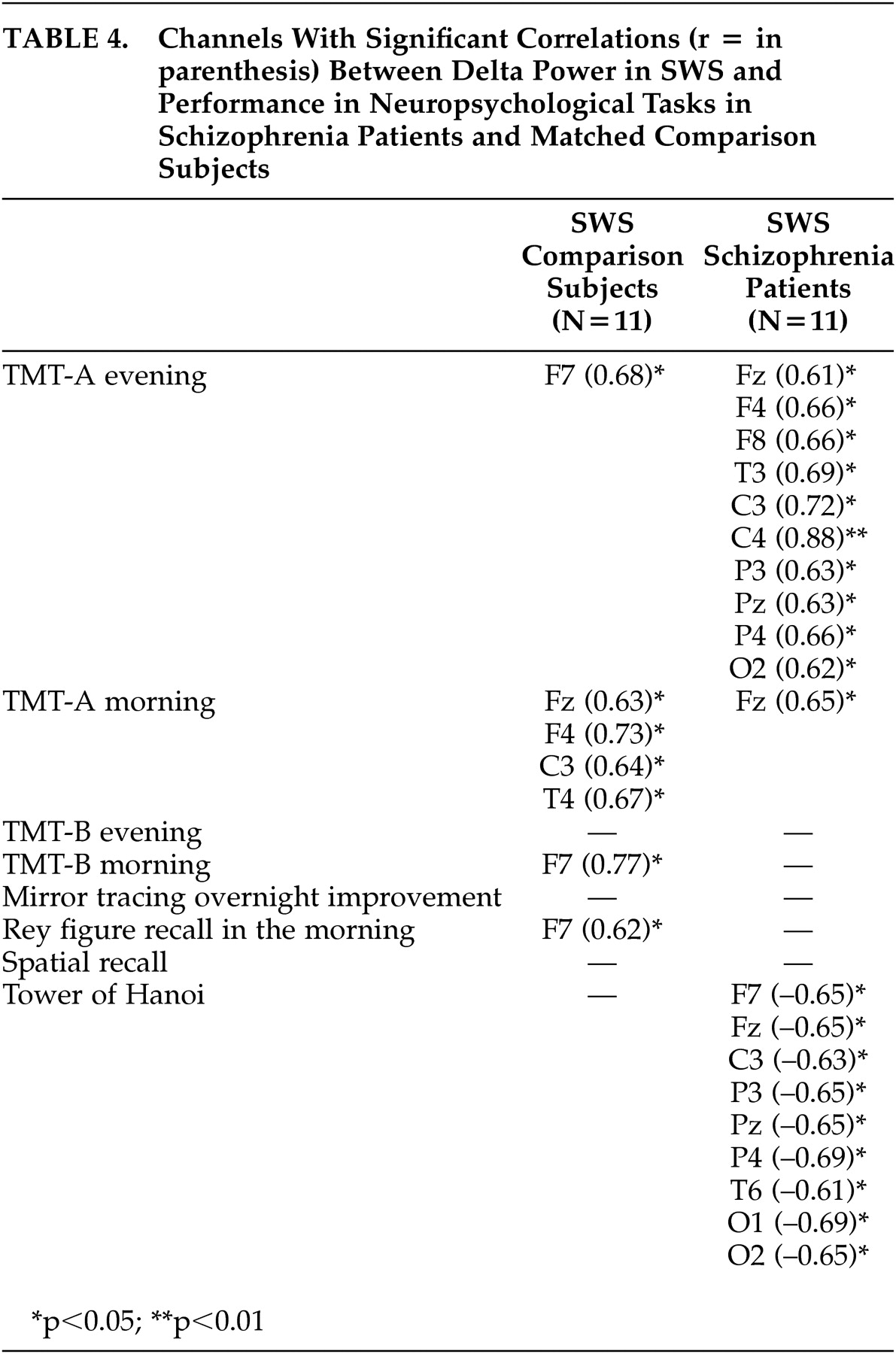
To our knowledge, this is the first report studying the interrelationship of delta power in sleep and neuropsychological performance in healthy subjects and schizophrenia patients . For healthy subjects, the main findings were significant positive correlations between performance in declarative memory, procedural learning, and attention/cognitive flexibility with delta power, especially in SWS and in frontal channels. In schizophrenia patients, significant positive correlations were only seen between delta power and performance in attention/cognitive flexibility. In addition, significant negative correlations were found to exist between performance in executive functions and delta power in sleep.
The findings of significant correlations between delta power in sleep and performance in neuropsychological tasks in healthy subjects are in line with recent published studies. Power in the delta or low delta range was also associated with performance in recall of the Rey figure in epileptic patients,
4 with performance in tests specific to the prefrontal cortex in older people
5 and with improvement in a procedural learning task in young healthy subjects.
6 Although Anderson and Horne
5 reported a strong link between frontal low delta power in non-REM sleep and a Tower test in old healthy subjects, we did not find this in young healthy subjects. This might be due to a different test procedure, because only a three-disk version applied to schizophrenia patients was preferentially sensitive to frontal lobe lesions, while the four-disk version used in our study was sensitive to basal ganglia disease.
17Because slow wave activity is the basis of non-REM sleep, it is not surprising that an association of performance in neuropsychological tasks was mainly found with delta power in SWS and Stage 2 sleep, but rarely in REM sleep. The highly significant correlation of performance in recall of the Rey figure and delta power in SWS in healthy subjects supports earlier studies that emphasize the importance of SWS versus REM sleep in the consolidation of declarative memory.
18 The association of improvement of performance in a mirror tracing skill with delta power in SWS and Stage 2 sleep confirms the importance of non-REM sleep also in consolidation of procedural memory.
3 The predominance of significant results in frontal channels in healthy subjects in our study is in line with other studies emphasizing the importance of frontal regions for performance of the Trail Making Test
19 and for declarative memory processes.
20,
21It was demonstrated by intracellular recordings in cats that certain oscillations in SWS enhance the responsiveness of thalamic and cortical neurons and it was therefore assumed that SWS may consolidate memory traces acquired during wakefulness in corticothalamic networks.
22 The intense discharge of neocortical neurons during the depolarization phase of the slow oscillations might provide signals that are used in synaptic reorganization, plasticity and the consolidation of information acquired in waking.
22,
23 Therefore, it was assumed that slow oscillations reflect a cortical reorganization of waking circuits
5 and might help synaptic consolidation.
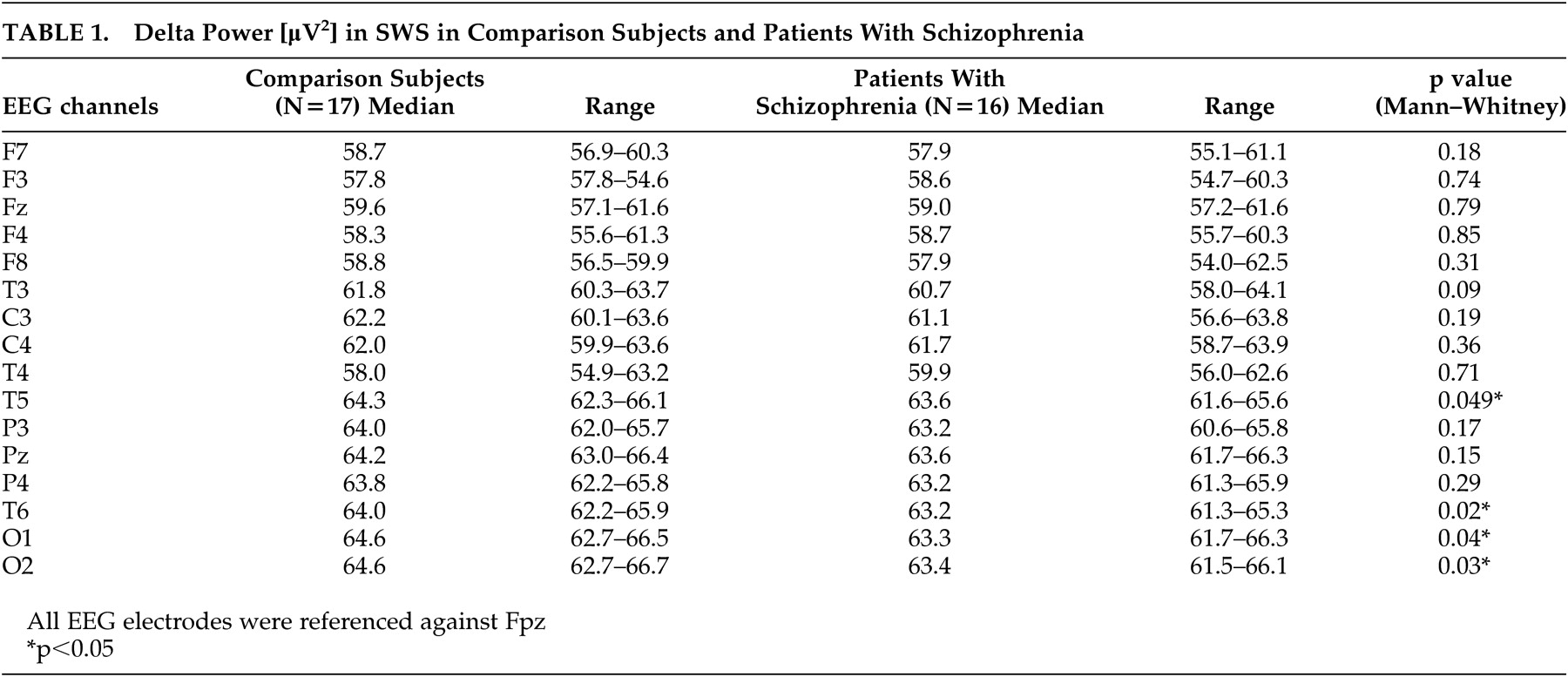
6In this study we analyzed the frequency range of 0.5 to 4 Hz, because in schizophrenia patients a decrease in classical delta activity in SWS was shown,
9 which was confirmed by our results. Regarding the known origin of delta waves within the thalamus and within the cortex, Keshavan et al.
9 assumed a thalamocortical dysfunction in schizophrenia. In line with this, a large number of papers are showing anatomical and functional alterations of the thalamus in schizophrenia.
21,
24,
25Cognitive deficits, considered to be key features of schizophrenia since the first descriptions of this disorder, are often linked to prefrontal dysfunction, also a consistent finding in schizophrenia research.
21,
26,
27 According to our hypothesis, we confirmed the results of a decrease in delta power in SWS. Additionally, the associations between especially frontal delta power and neuropsychological morning performance in healthy subjects were not found in patients with schizophrenia. Because a decrease in delta power in patients was only seen in temporal and occipital, but not frontal channels, it might be speculated that the above-mentioned link between delta activity and synaptic consolidation and plasticity
22,
23 was additionally disturbed in schizophrenia patients.
Our results concerning the schizophrenia subjects are of course limited by the fact that our patients were not drug-free. Systematic data on the impact of amisulpride on delta power in sleep in schizophrenia have not yet been published. But in our previous paper
7 we reported typical alterations, especially of non-REM sleep parameters in our patients on stable medication with amisulpride as known from the literature of drug-naïve patients.
8 Furthermore, we did not find significant correlations between the dosage of amisulpride and delta power in any EEG channel. Because our patients, as opposed to comparison subjects, also showed diminished delta power in SWS, as did drug-free patients,
9 our patients seem to be quite typical in this respect.
In conclusion, we found further indications for the importance of especially frontal delta oscillations for neuropsychological performance in healthy subjects. Correlations cannot delineate causal relationships, but a diminution of this link might indicate disturbances in thalamocortical and prefrontal networks in schizophrenia. Future studies need to confirm and to extend these pilot results in a greater number of subjects. It should be analyzed further if applications of delta activity-enhancing drugs, such as gaboxadol,
28 could yield positive correlations between delta power and neuropsychological performance in schizophrenia patients. Finally, we believe that spectral analysis of sleep EEGs might provide new insights into processes of cognitive dysfunctions in a complex disorder like schizophrenia.