P atients with epilepsy are more prone than the general population to psychiatric disorders, being especially susceptible because of psychological reasons (e.g., disruption of social functioning, stigma, frustration and fear of seizures), iatrogenic factors (e.g., psychotropic effects of antiepileptic drugs), and neurobiological variables (e.g., severity, type and localization of epilepsy).
1 Aura phenomena may indicate electrophysiological disruption in specific anatomic areas, providing useful information with respect to the region of the brain where seizures start. Actually, auras demonstrated a high localizing but not lateralizing value.
2 Some authors investigated the relationship between aura and psychopathology, hypothesizing that patients with experiential phenomena, such as fear, déjà vu, jamais vu, illusions and vivid hallucinations, were more at risk of developing psychopathology. This hypothesis relates to the fact that experiential auras may suggest the involvement of limbic structures, especially the amygdala.
3 –
6 All studies agree that experiential auras in patients with drug-resistant epilepsy are associated with a high prevalence of depression and anxiety.
4 –
6 This is in line with the role played by the amygdala and mesolimbic structures in anxiety and depressive disorders.
Dissociative experiences may occur on a continuum of situations, from healthy individuals, often under conditions of stress, fatigue, or drug use, to a severely debilitating disorder where the symptoms can persist chronically and unremittingly for decades. They can occur in a number of neurological disorders (e.g., epilepsy and migraine), but mainly occur in psychiatric disturbances, such as anxiety disorders, especially panic disorder, and major depression.
7 In this study we investigate the relationship between aura, psychopathology, and dissociative experiences in patients with epilepsy, and try to discern the extent to which dissociative experiences are true epileptic phenomena or are related to anxiety or other comorbid psychiatric disorders.
METHOD
All adult patients with a diagnosis of temporal lobe epilepsy (TLE) admitted to the epilepsy clinics of the Department of Neurology, Amedeo Avogadro University in Novara, Italy, during 2003, were asked to be enroll in the present study. The epilepsy diagnosis was performed according to the International League Against Epilepsy criteria
8 (clinical features, EEG or videoEEG and neuroimaging) and performed by at least two different neurologists not involved in the present study.
We excluded patients younger than 18 years of age, and those with a diagnosis of pseudoseizures, with an uncertain diagnosis of TLE or the presence of extratemporal foci, with a grade reading level less than 6th, with learning disabilities or mental retardation and, in general, patients with a Mini-Mental State Examination (MMSE) <24. After an explanation of the procedures, all subjects gave informed consent.
The auras were typed by a neurologist (Dr. Collimedaglia) on the basis of the Montreal, Neurologic Institute classification of epileptic auras.
2 Psychic auras were defined as cognitive (dreamy states=mixed dream and present reality; forced thinking=recurrent and stereotyped intrusive thoughts; depersonalization=sense of unreality about the self; derealization=sense of unreality about the external world; altered time sense=distorted perception of time) and noncognitive (affective=fear, anxiety and so on; illusion=modification in perception of objects, e.g., macropsia or metamorphopsia; dysmnesic=déjà vu, jamais vu; dysphasic=speech arrest or impaired comprehension; hallucinations=visual experiences).
2 –
5 In addition to psychic auras, the other aura categories were classified according to a modified version of previously adopted classifications (visceral=epigastric rising sensation; cephalic=head pressure; somatosensory=tingling sensations, numbness and other sensations; taste/smell=smells and tastes; elementary visual=bright lights, visual blurring; warm=warmth over the trunk; elementary auditory=tinnitus; confusion=mixed-up thoughts).
2,
5 There was complete agreement on aura typing as performed by the study neurologist and as originally reported by the neurologist in the clinics.
All subjects were evaluated by a fully trained clinical psychologist (Dr. Magli) under the supervision of a neuropsychiatrist (Dr. Mula).
The psychological assessment included the Minnesota Multiphasic Personality Inventory 2 (MMPI-2),
9 the Beck Depression Inventory (BDI),
10 the State-Trait Anxiety Inventory Y1 and Y2 (STAI Y1 and Y2)
11 and the Dissociative Experiences Scale, version II (DES-II)
12 considering a clinical threshold of 10. All tests were administered in a standardized way and in the same sequence to all patients. MMPI-2 was scored by a computer using Psy-System II (version for Windows, Florence, Italy). Row scores on each scale were converted to uniform T scores which have the same percentile and are corrected for the K scale. MMPI-2 profiles were considered valid if fewer than 15 items were omitted. Data included in the analysis were K-scale-corrected T scores. Scores >65 were considered pathological.
Initially, we compared MMPI-2 profiles, BDI, STAI Y1 and Y2 and DES scores of patients with and without aura and subsequently those of patients subdivided for aura subtype (psychic versus nonpsychic; cognitive versus noncognitive and nonpsychic). We also investigated the same parameters comparing patients with and without drug-resistant epilepsy, subsequently subdivided for aura type.
Moreover, we analyzed DES scores and subscales (absorption/imaginative involvement, amnesia and depersonalization/derealization), segregating patients for presence of aura, aura subtype, drug resistance, EEG laterality (right/left, monolateral/bilateral, anterior/posterior). Finally, we correlated DES scores with pertinent subscales of MMPI-2, BDI and STAI Y1 and Y2 to identify which psychological determinants may be relevant.
All groups were also compared for the following items: age, gender, personal history of psychiatric disorder (classified as mood disorder, psychotic disorder, personality disorder, behavior abnormalities or others), age at onset of epilepsy, duration of epilepsy, seizure frequency (classified as seizure-free, 1 to 10, 11 to 20 or >20 seizures/month), MRI features, laterality and localization of EEG epileptic abnormalities, and anticonvulsant drug regimen and combinations.
We used χ 2 analysis or Fisher’s exact test for categorical data, and assessed ordinal and linear data by nonparametric tests and one-way analysis of variance. Given the number of analyses, a Bonferroni’s correction was applied, with differences considered significant at p<0.001. Linear relationships have been tested using the bivariate correlations procedure and, considering the number of patients investigated, we chose two-tailed nonparametric measures with p<0.05.
Statistical analysis was performed using the Statistical Package for Social Sciences (Version 12 for Windows, SPSS Inc., Chicago, Ill.).
RESULTS
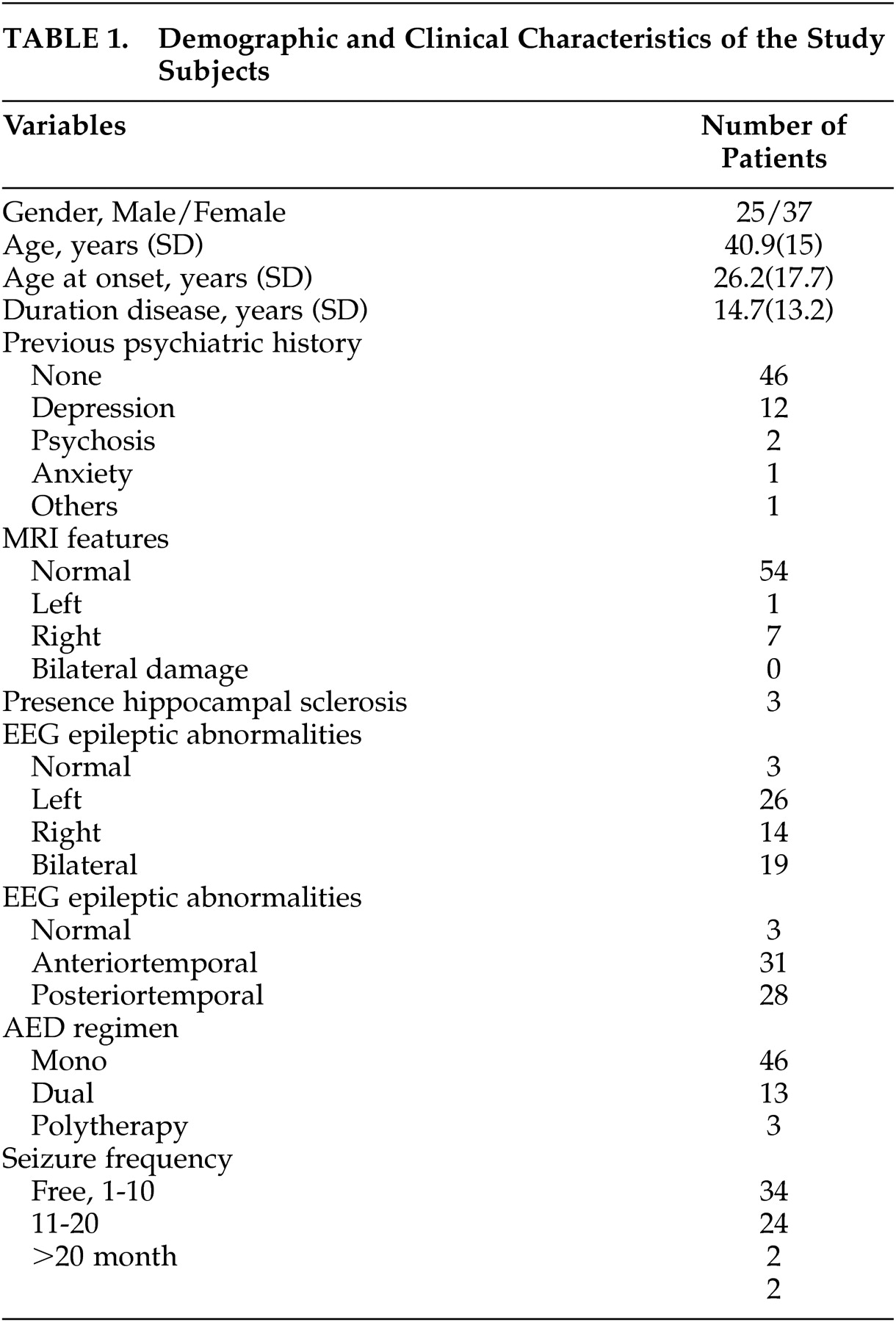
We recruited 62 patients with a diagnosis of TLE (25 men), a mean age of 40.9 (SD=15) years, and duration of the disease lasting 14.7 (SD=13.2) years. Demographic and clinical characteristics of the population investigated are shown in
Table 1 . Fifty-four had a diagnosis of cryptogenic TLE and only eight had symptomatic epilepsy (three with hippocampal sclerosis). Thirty-four (54.8%) patients were seizure-free, 24 (38.7%) experienced one to 10 seizures/month, two (3.2%) experienced 11 to 20/month, and two (3.2%) experienced more than 20 seizures/month. Forty-five (72.6%) patients were taking one medication (61.3% with carbamazepine), 13 (21%) in bitherapy and four (6.4%) in polytherapy. Twenty (32.3%) patients were classified as having drug-resistant seizures.
Type and frequency of auras are shown in
Table 2 . Thirty patients (48.4%) reported at least one aura but most of them had more than one (range=1 to 3). There were no significant differences in age, gender, education level, age at onset and duration of the epilepsy, seizure types and frequency, antiepileptic drug regimen and combinations.
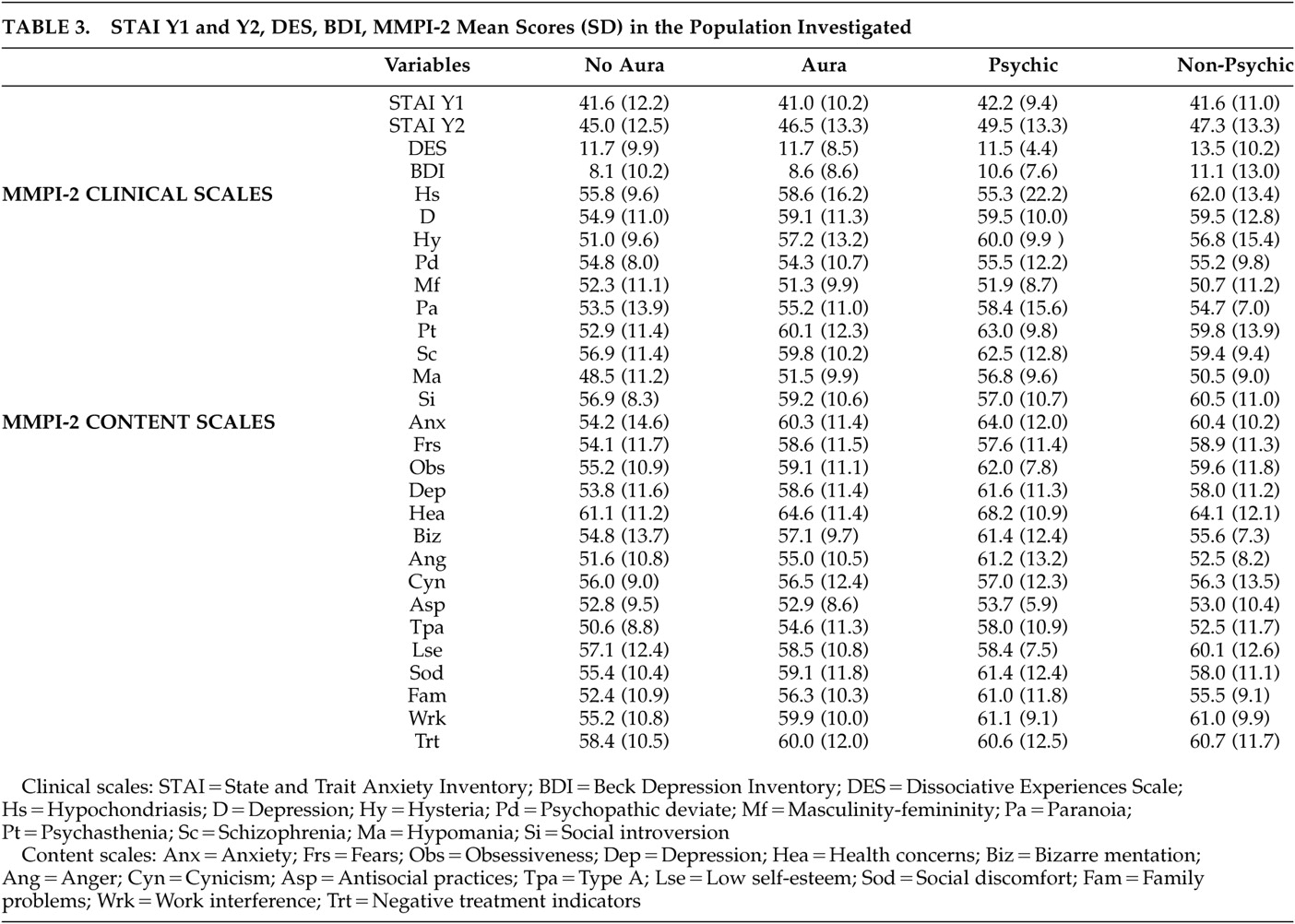
There was no difference in MMPI-2 profile, BDI, STAI Y1 and Y2, DES (total and subscale) scores comparing patients with and without aura, psychic auras versus nonpsychic, aura subtypes (cognitive versus noncognitive versus nonpsychic) and drug-resistant versus seizure-free patients (relevant data are summarized in
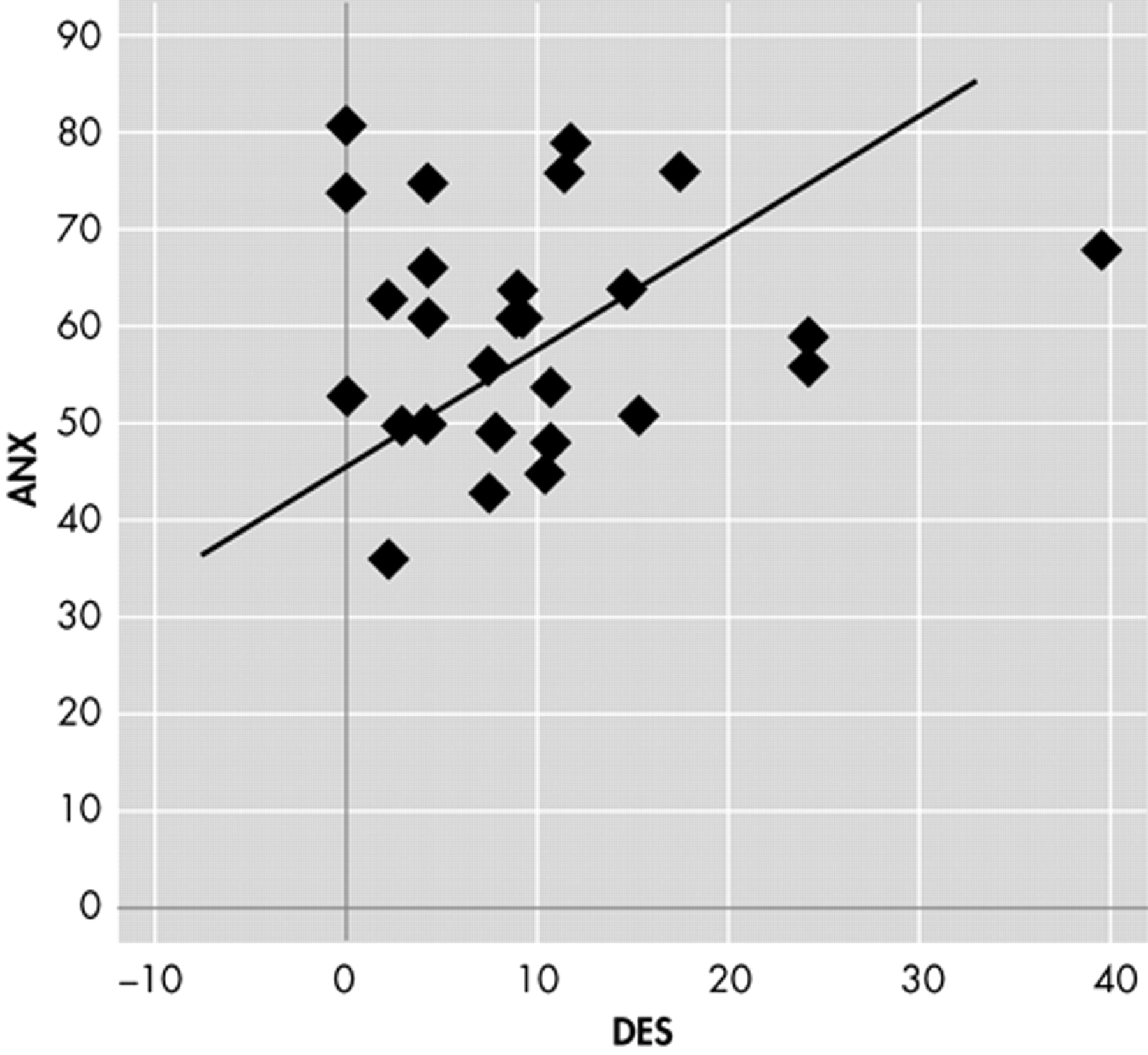
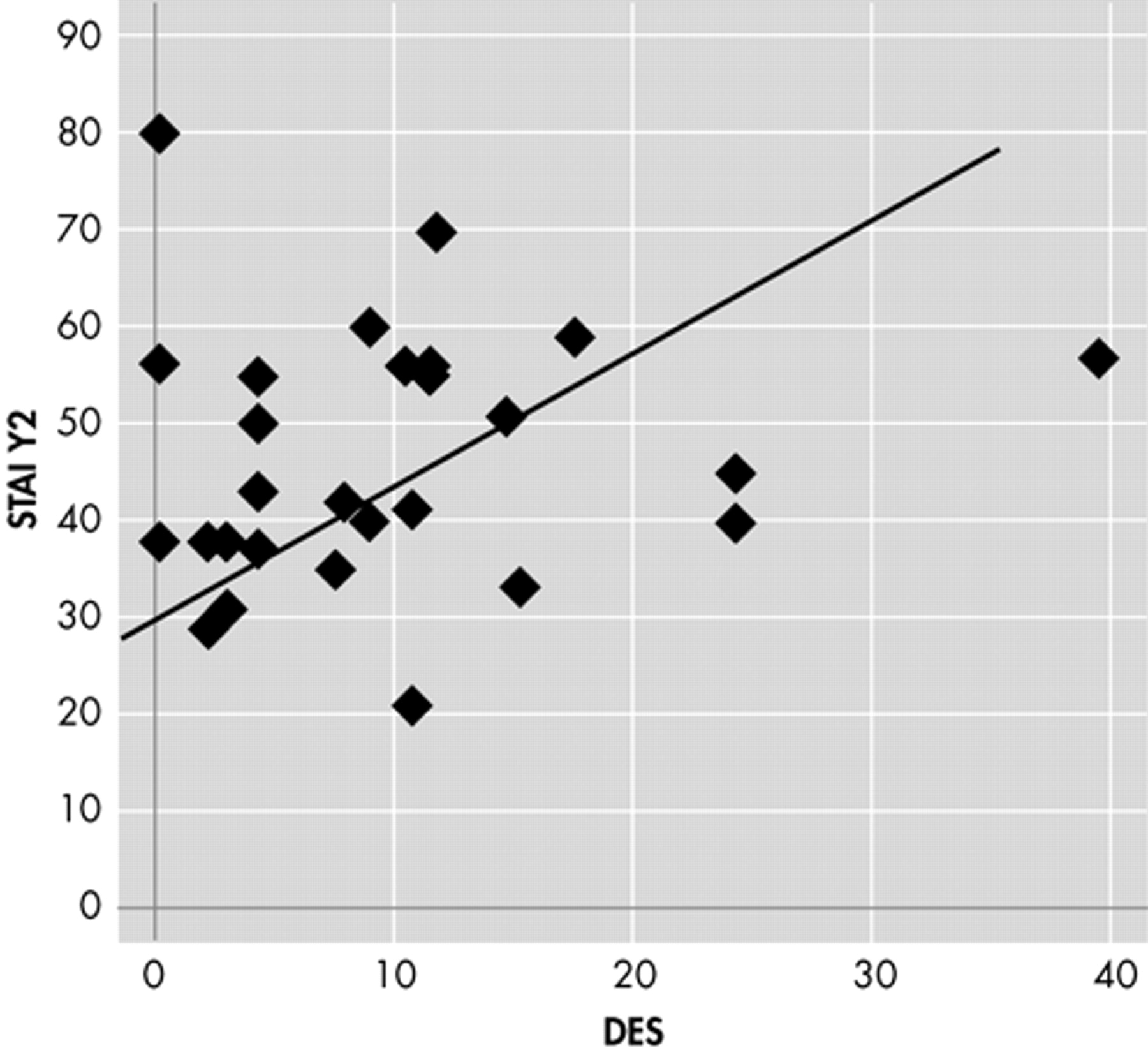
Table 3 ). Moreover, we observed no difference in DES scores and subscales segregating patients for EEG laterality (right/left, monolateral/bilateral, anterior/posterior). Nine patients had a DES score >10 but we found no difference in MMPI-2 profile, BDI, or STAI Y1 and Y1 scores when compared to other TLE patients with low DES scores. Bivariate correlation showed a linear relationship between DES and ANX subscale of MMPI-2 (r=0.388, p<0.05) and STAI Y2 (r=0.390 p<0.05) only in patients with aura (
Figures 1 and
2 ).
DISCUSSION
Several authors have reported a prevalence of aura ranging between 20% and 81% in patients with focal epilepsy
2,
6,
13 –
16 and 35% and 93% in patients with TLE with a mean of 67.3% and median of 70% when calculated comparing the eight major studies.
2,
16 –
22 In our sample, the prevalence of aura in general was 48.4%, in line with some studies but slightly under the mean. This might be due to a selection bias in other investigations. In fact, most of the studies have been performed in tertiary referral centers or assessed patients refractory to medical treatments who were candidates for epilepsy surgery. A large study of 666 nonsurgical TLE patients reported a prevalence of auras of 49%.
17 As far as different aura subgroups are concerned, we observed that the most frequently reported auras were nonpsychic (45.9%), especially epigastric ones (19.3%). Although our rates are considerably lower than those reported by Palmini and Gloor (37%),
2 Fried et al. (42%),
23 Henkel et al. (52%),
24 they are in keeping with other authors who reported 14%,
15 15%,
19 and 21%.
20 This may be due to the same reasons discussed above. Therefore, prevalence and distribution of auras in our population are in keeping with the literature.
The relationship between epilepsy and psychopathology is complex, and some studies have investigated the role of auras.
3 –
6,
25 –
28 We agree that the presence of aura per se does not represent a relevant factor for the occurrence of psychopathology while experiential or psychic aura may be associated with a wide spectrum of psychopathology, such as depressive traits and anxiety with psychosocial difficulties,
4,
5,
28 psychotic features,
3,
26 or personality disorders.
6,
26 However, looking at previous studies, the presence of psychopathology seems to be related to the temporal lobe itself, and the association with experiential auras is probably due to their relatively higher prevalence in TLE than in other focal epileptic syndromes.
6 Our results are in line with previous studies and confirm that there are no differences in the psychopathological profile of patients with and without aura, or with different aura subtypes, considering only patients with TLE.
Another interesting issue is the relationship among dissociative experiences, aura, and psychopathology. Dissociative amnesia, depersonalization, derealization, déjà vu, and jamais vu occur on a continuum of situations, from healthy individuals to those with neuropsychiatric disorders. Although the interest in their phenomenology and epidemiology has flourished over the last decades with the accumulation of a large literature, they have been only occasionally investigated in patients with epilepsy because researchers focused their attention mainly on patients with pseudoseizures. The only comparable study is the one by Devinsky et al.,
29 which assessed 71 epileptic patients with the DES and compared them with healthy subjects and a small group of subjects with multiple personality disorder. The authors showed that DES scores of the seizure patients were moderately elevated compared with those of healthy individuals, but similar to those previously reported for psychiatric patients with anxiety or phobic disorders. However, they did not clarify whether these phenomena might be truly epileptic or due to the presence of a comorbid anxiety disorder, or both. In our study, we observed no difference in DES scores in patients with or without auras or in different aura subtype (especially psychic), suggesting that dissociative experiences are not likely to be related to the aura itself. However, it is interesting that DES scores correlated with the anxiety subscale (ANX) of MMPI-2 and STAI Y2 score (measuring state anxiety) only in patients with aura. Both scales usually refer to baseline anxiety levels and correlate with the presence of subclinical features or a clinical diagnosis of anxiety disorder. All this evidence taken together might suggest that dissociative experiences in epilepsy rarely represent true epileptic phenomena but are usually associated with anxiety. Our findings have theoretical relevance in light of the present view about dissociation in epilepsy.
30 The notion that many epileptic phenomena can be regarded as dissociative is often based on the occurrence of amnesia,
31 but several manifestations associated with partial seizures, such as cognitive auras, déjà vu, and déjà vécu, should be distinguished from true episodes of dissociation. Auras probably originate from the activation of representational structures in the temporal lobes, either directly by seizure activity in representational networks, or indirectly through seizure-related stimulation of limbic structures, such as the amygdala and the anterior cingulate.
32 Our results suggest that dissociative experiences in epilepsy are often related to comorbid anxiety and should not be easily inferred as epileptic in nature without considering possible underlying psychopathological and biological factors. Actually, auras per se, especially psychic ones, represent unusual experiences, often with a distressing nature, making patients more “conscious” about their seizures. Moreover, these auras suggest the involvement of specific structures in the limbic system, with an increased risk to develop anxiety or affective disorders according to a kindling paradigm.
33 Therefore, patients with aura may be more at risk of developing anxiety disorders, which represent the basis for the occurrence of dissociative experiences.