METHOD
The study was a parallel group, sham-controlled, double-blind trial. Forty-five patients with unipolar major depressive disorder (SCID and DSM-IV) were randomized to four groups.
Group 1. High frequency left-sided DLPFC rTMS (N=10)
Group 2. Low frequency left-sided DLPFC rTMS (N=10)
Group 3. Low frequency right-sided DLPFC rTMS (N=10)
Group 4. Sham rTMS (N=15)
Group 4. (Sham rTMS) was subdivided into three further groups corresponding to the sites of real stimulation:
Group 4a. High frequency left-sided DLPFC sham rTMS (N=5)
Group 4b. Low frequency left-sided DLPFC sham rTMS (N=5)
Group 4c. Low frequency right-sided DLPFC sham rTMS (N=5)
High frequency right-sided DLPFC rTMS was not included in this study, given the negative results of this intervention in a previous study.
15The patients in our study had all been referred for ECT having failed an adequate course of antidepressant medication.
The patient characteristics are summarized in
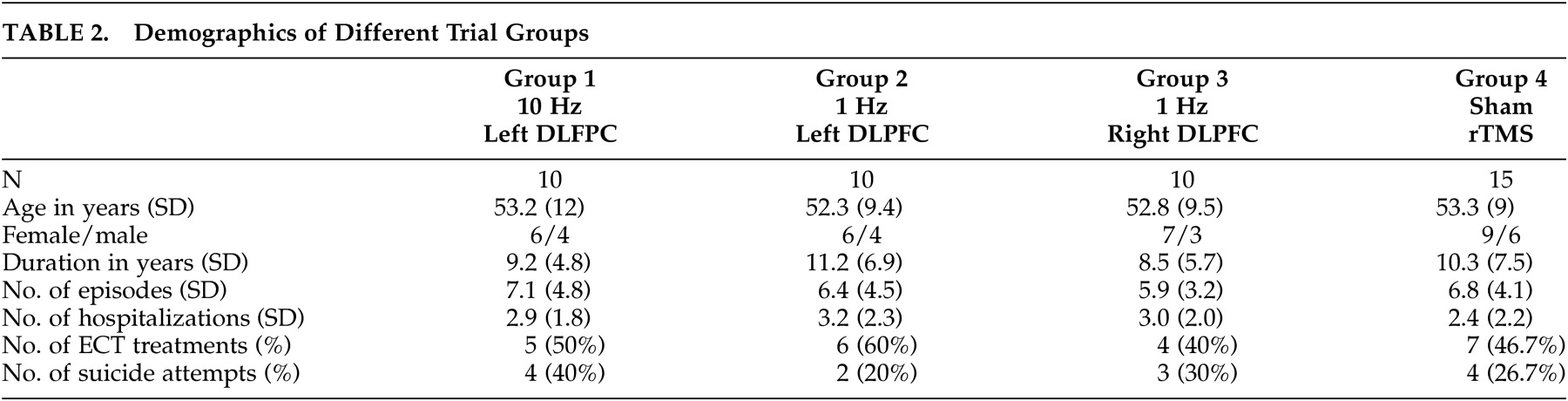
Table 1. The number of patients with a history of hospitalization, ECT, or suicide attempts is relatively high compared with the general population of patients with major depressive disorder, suggesting that these patients are at the sicker end of the spectrum.
All patients were right-handed (Oldfield questionnaire) and between the ages of 21 and 80. Patients were interviewed by a psychiatrist who was able to consult with their treating psychiatrist, and they met DSM-IV criteria for a major depressive episode with a score ≥20 on the 21-item Hamilton Depression Rating Scale (HAM-D).
They had no psychotic features and no other Axis I disorders. All patients were naïve to TMS, and none had participated in previous research studies on TMS and depression. All patients were treated as outpatients. The study was approved by the Institutional Review Board. Written consent was obtained from all participants. Patients then underwent a 14-day washout of all psychotropic medication, and the reinstatement of this medication was not permitted until the protocol was completed. PRN Lorazepam (maximum of 2 mg per day) was allowed during the first 7 days of washout. Patients unable to tolerate the medication withdrawal were excluded.
Exclusion criteria included a history of any psychotic disorder, including schizophrenia or schizoaffective disorder; bipolar disorder; obsessive compulsive disorder; personality disorder; substance abuse (except nicotine) within past year; current acute or chronic medical condition requiring treatment with psychoactive medication; a history of epilepsy or unprovoked seizures or other neurological disorder; abnormal neurological examination; family history of medication-resistant epilepsy; prior brain surgery; metal in the head; an implanted medical device; or pregnancy.
rTMS sessions were conducted at a laboratory staffed by personnel certified in basic life support and trained in the prompt recognition and treatment of seizures and other medical emergencies. A physician was always present during the treatments. Emergency equipment such as oxygen, IV access tools, and emergency medication was available.
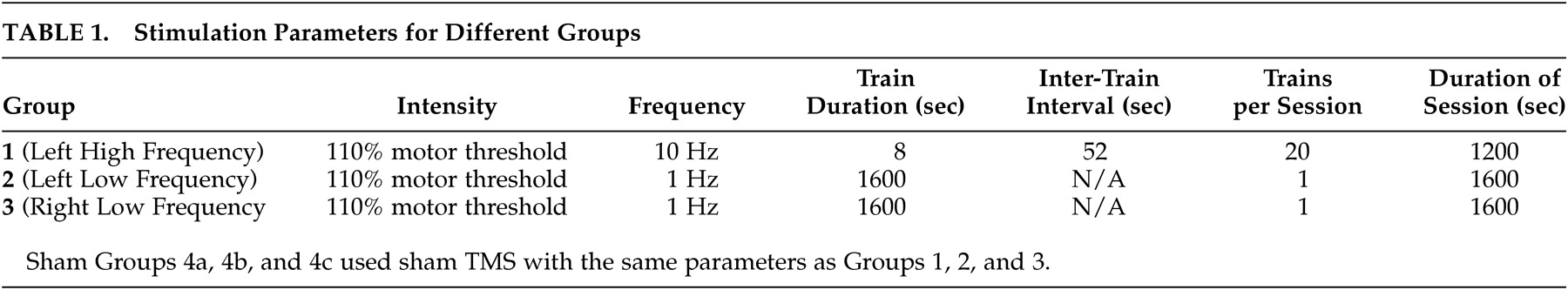
We used a Dantec Magpro magnetic stimulator (Dantec Medical Inc, Denmark) and a Magstim Super-Rapid Magnetic Stimulator (Magstim Corporation, UK). Both used an identical 8-shaped focal stimulation coil. These machines were used randomly between patients, with each patient receiving stimulation from only one machine. Treatment parameters were based on motor threshold and were thus independent of any intermachine variability. Sham TMS was delivered by orienting the coil perpendicularly to the patient’s scalp, while in the treatment conditions the coil was held tangentially to the scalp. During each treatment, the patient’s head was held with a head support and coil position was fixed using a coil holder.
Motor threshold was determined on the first day of treatment in accordance with the recommendations of the International Federation of Clinical Neurophysiology.
19Surface EMG electrodes were used to record from the belly of the right abductor pollucis brevis (APB) muscle. Using suprathreshold stimulation, the stimulation site producing the maximal EMG response was identified. A descending staircase technique was used to determine the threshold for APB activation. The threshold was defined as the lowest stimulation intensity to produce MEPs of at least 50uV amplitude (peak to peak) in at least five out of 10 trials.
As in previous studies,
15 stimulation was applied to an area 5 cm anterior of and in the same parasagittal plane as the site producing the maximal EMG response in the contralateral APB muscle. This is presumed to have targeted the middle third of the frontal gyrus, approximately the border of Broca’s areas 46 and 9. Fitzgerald et al.
18 used the same landmarks in their study.
The stimulation parameters for the different groups are shown in
Table 1.In compliance with current safety guidelines,
20 patients and TMS technicians wore earplugs to prevent possible auditory threshold shifts. All sessions, including sham, were conducted under continuous video monitoring to allow for detailed evaluation of any unexpected complication. Patients completed a side effect checklist before and after each TMS application. The parameters for Group 1 (
Table 1 ) were slightly above the recommended safety guidelines, which recommend a maximum intensity of 80% motor threshold. However, our growing body of experience
1 suggests that these parameters are safe. Patients were made aware of the limited availability of safety data during the process of informed consent.
A psychiatrist blinded to group assignment conducted all assessments of patients’ symptoms. He was able to discuss the cases with the patients’ treating psychiatrists but remained blinded to group assignment. Patients were evaluated following the 14-day medication withdrawal period and weekly thereafter using the 21-item HAM-D.
21 In accordance with many other studies on depression, a clinically significant response was defined as a HAM-D reduction ≥50% relative to pretreatment baseline. Remission was defined as a HAM-D score ≤10. All subjects had a baseline score of ≥20 on the HAM-D (21 item); therefore all patients fulfilling the criteria for remission also fulfilled the criteria for clinical response.
RESULTS
Demographics
Baseline patient characteristics of the different study groups were compared using a one-way analysis of variance (ANOVA) which confirmed comparable study populations.
Table 2 summarizes the patient characteristics of the four different groups. There were no significant differences in age, gender, handedness (Oldfield Inventory Scores), duration of major depressive disorder, number of depressive episodes, number of hospitalizations for depressive episodes, or suicide attempts or prior ECT.
There was no significant difference between groups in baseline HAM-D score (F [3, 41]=0.02, p>0.05) although there was some variability between individual subjects. To minimize this, results were analyzed using percentage decrease from baseline HAM-D score in each subject.
HAM-D Scores
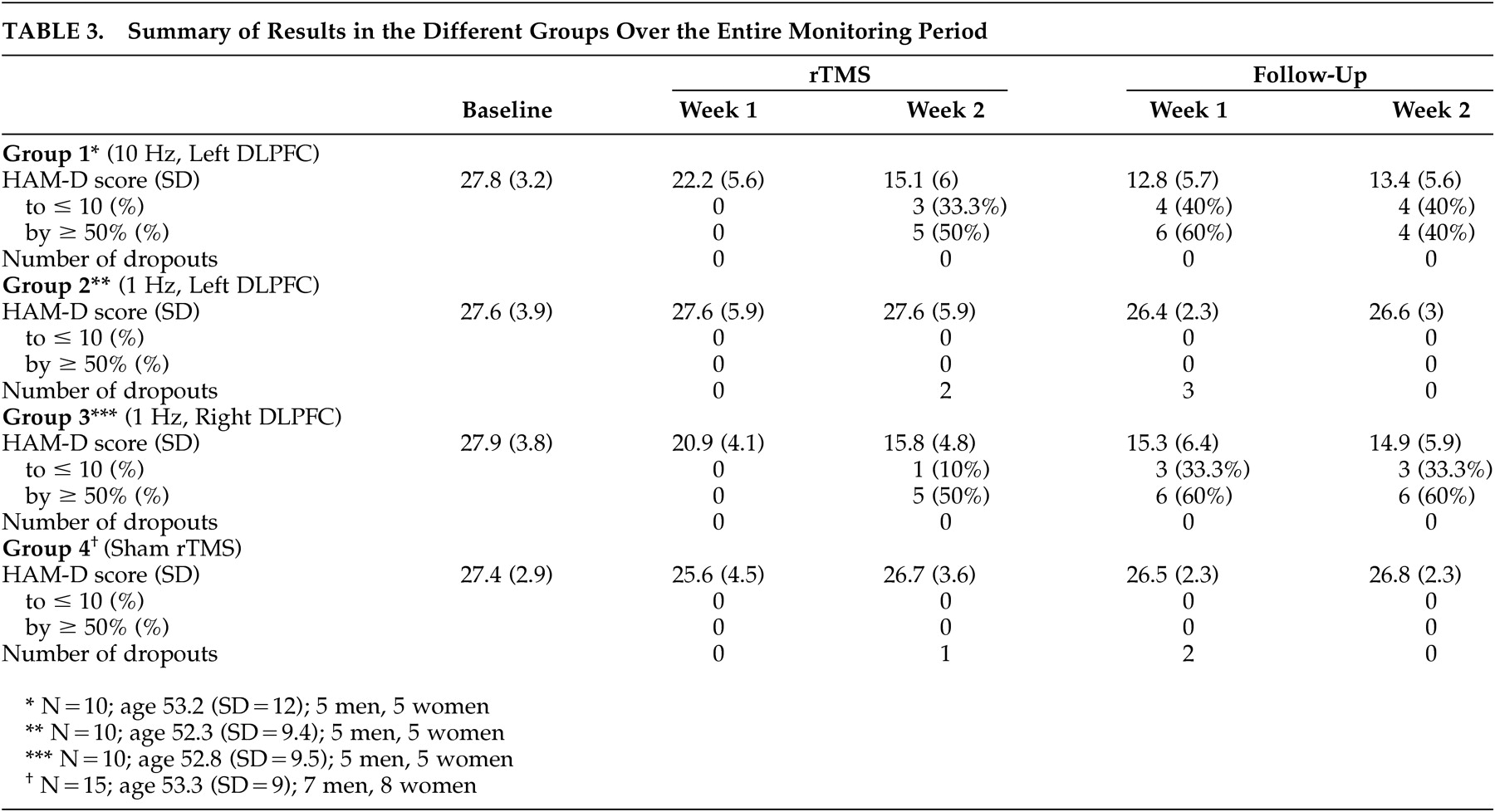
None of the patients in Group 2 (left-sided, low frequency rTMS) or Group 4 (sham rTMS) met the criteria for a clinical response. However, at the end of the 10 days of stimulation, 60% of the patients in Group 1 (left-sided, high frequency rTMS) and 60% in Group 3 (right-sided, low frequency rTMS) showed a clinical response. Similarly, while none of the patients in Group 2 or Group 4 met the criteria for remission, 33.3% of the patients in Group 1 and 10% of the patients in Group 3 showed this level of improvement (
Table 3 ).
All patients were followed up for 2 weeks after the treatments ended. The number of patients in remission in both Group 1 and Group 3 increased slightly in the week following treatment (from five to six). In Group 1, the number of patients in remission had fallen to four by Week 2 of follow-up, representing an increase in HAM-D score above the threshold for remission for two patients. The difference between Group 1 and Group 3 at this stage was not statistically significant. The patients in Group 1 and Group 3 had their HAM-D scores reevaluated 4 weeks after treatment. Since only the active groups were followed up, this must be regarded as an unblinded follow-up. Four out of 10 patients in Group 1 and two out of 10 patients in Group 3 still met the criteria for a clinical response. The average decrease in HAM-D score between baseline and 4 weeks posttreatment was 40% in both groups.
Statistical Analysis
A mixed design factorial analysis was performed between the four groups over the four time points. Significant changes were found between both the different groups (10 Hz left, 1 Hz left, 1 Hz right, and Sham: F [3]=25.70, p<0.0005) and the different time points (rTMS–1, rTMS–2, Week 1, Week 2: F [3]=14.72, p<0.0005).
Part A: Analysis of Differences Within Each Group Over Time
Group 1 HAM-D scores differed significantly over time (F [3, 9]=47.22, p<0.0005). A post hoc modified Bonferroni analysis for all possible comparisons yielded significant differences (p<0.01) in HAM-D change scores between rTMS–Week 2, Follow-up Week 1, and Follow-up Week 2, when compared to rTMS–Week 1.
Group 2 HAM-D scores did not differ significantly (p>0.05) over time when all time points were compared.
Group 3 HAM-D scores differed significantly over time (F [3, 9]=21.49, p<0.0005), like Group 1. Here, a post hoc modified Bonferroni analysis for all possible comparisons also yielded significant differences (p<0.05) in HAM-D change scores between rTMS–Week 2, Follow-up Week 1, and Follow-up Week 2, when compared with rTMS–Week 1.
Group 4 HAM-D scores did not differ significantly (p>0.05) over time when all time points were compared.
Part B: Analysis of Differences Between Groups Over Time
HAM-D percent change scores were significantly different between the groups at all time points after baseline (rTMS–Week 1, p=0.018; rTMS–Week 2, p=0.0001; Follow-up Week 1, p=0.0001; Follow-up Week 2, p=0.0001).
These results were broken down using a post hoc modified Bonferroni analysis. Group 1 differed significantly from both Group 2 and Group 4 at time points rTMS–Week 2, Follow-up Week 1, and Follow-up Week 2 (all p<0.0005). We found similar results for Group 3, with significant differences compared to Group 2 and Group 4 found at time points rTMS–Week 2, Follow-up Week 1 and Follow-up Week 2 (all p<0.0005). There were no differences between Group 2 and Group 4 (sham) at any time point. Also, there were no differences between Group 1 and Group 3 at any time point.
Finally, we found no correlation between baseline HAM-D scores, gender, age, or prior history of hospitalizations or ECT and subsequent improvement.
Withdrawals and Adverse Effects
A total of eight patients withdrew from the study due to adverse effects. These patients were from Group 2 (five patients) and Group 4 (three patients). No patients withdrew from Group 1 or Group 3, which were the groups showing a significant antidepressant effect. All subjects tolerated the procedure without any major complication; specifically, none of the patients experienced a seizure.
One patient complained of severe headaches on four occasions during the 10 days of rTMS and was prescribed analgesics (acetaminophen or aspirin). This patient requested release from the study at the end of the second week of rTMS. This patient was in Group 4a, receiving sham, left-sided high frequency rTMS.
While no other patients required analgesia, nine of the 45 patients reported headaches which they rated subjectively as “severe” on at least one of the days of rTMS. These headaches always subsided without treatment within a few hours of rTMS. There was no correlation between headaches and eventual antidepressant effects.
Optimal Stimulation Parameters for rTMS Treatment of Depression
The findings of this study confirm the results of previous sham-controlled trials of rTMS in depression
14,
15 in finding antidepressant effects of rTMS in patients with major depressive disorder. Both high frequency left-sided rTMS and low frequency right-sided rTMS led to a clinically significant antidepressant effect (≥50% reduction in the HAM-D score) in 60% of patients. The magnitude of this effect is similar to the results in our previous trial
15 and greater than some other published reports.
22 This difference in degree might be related to differences in the rTMS parameters. In our studies, we have used both a higher stimulation intensity (110% in the present study) and a larger number of stimuli per session (1,600 in the present study) than most other reported studies. These parameters are now widely considered safe.
1The main result of the present study is that low frequency rTMS to the right DLPFC has similar antidepressant effects to high frequency rTMS to the left DLPFC. These results confirm the findings of Fitzgerald et al.
18 In our previous sham-controlled trial of rTMS in depression,
15 right DLPFC rTMS at high frequency was found to lack antidepressant effects (and for this reason, this was not included as a treatment arm of the current study). In the present study, low frequency rTMS to the left DLFPC did not show antidepressant activity.
Though our study was not designed to assess the duration of the antidepressant effect of rTMS, it is worth noting that our unblinded follow-up at 4 weeks appeared to show an equal duration of antidepressant effect for both high frequency left-sided rTMS and low frequency right-sided stimulation.
If it is accepted that high frequency left-sided rTMS and low frequency right-sided stimulation are equally effective in the treatment of depression, factors other than efficacy must be taken into account when selecting a treatment modality. The most serious potential side effect of rTMS is seizure, and a growing body of evidence suggests that low frequency rTMS may be protective against seizures.
23 Therefore, low frequency rTMS should be the treatment of choice for patients with risk factors for epilepsy. On the other hand, high frequency treatment is better researched at present and may remain the first-line modality for some time.
Ultimately, it remains unclear whether some patients respond better to high freqeuncy left-sided rTMS and others to low frequency right-sided rTMS. Analysis of our data fails to reveal any obvious differences between the patients responding to one or another rTMS regimen. It is possible that by increasing activity on the left side, one may transcallosally suppress activity on the right and vice versa. Therefore, the physiological effects of high frequency left-sided and low frequency right-sided rTMS may, in fact, be equivalent. Alternatively, some characteristics may predispose patients to respond to one or the other modality. Our study did not address this issue. Fitzgerald et al.
18 attempted to investigate this with a crossover design in their study. Nonresponders to either high or low frequency treatments from the first leg of their study were given the alternate treatment. The patients were unblinded during their second phase of treatment, and some of the improvements measured might have had a placebo effect or a delayed response to the first phase of treatment. Of 17 patients crossed over, only three responded to the alternate treatment. It is not possible to come to a conclusion on this issue without more data.
Another hypothesis that remains to be adequately tested is that left-sided high frequency treatment and right-sided low frequency treatment might have an additive effect. Fitzgerald et al.
24 showed that this approach has efficacy in the treatment of major depressive disorder, but they compared it with placebo rather than with unilateral treatment, making comparison difficult.