N atural opioids are derived from the plant Papaver Somniferum, commonly known as the opium poppy. The milky fluid extracted from the plant’s unripe seed capsule is highly narcotic (e.g., pain relieving as well as dulling to the senses) after drying. This characteristic has made for a history rich in medicinal value and political debate.
7 In 1680, the well known English apothecary, Thomas Sydenham, introduced Sydenham’s Laudanum, a compound containing opium, sherry wine, and herbs. Sydenham would later write the words: “Among the remedies which it has pleased Almighty God to give to man to relieve his sufferings, none is so universal and so efficacious as opium.”
8 In 1806, Sertürner isolated the active ingredient in opium.
7 He named the resulting alkaloid (principium somniferum) “morphine” after Morpheus, the god of dreams. Isolation of codeine (Robizuet 1832) and papaverine (Merck 1848) followed.
8 In the 1850s, following the introduction of the hypodermic syringe and hollow needle, physicians began administering morphine by injection.
7 The effect was instantaneous and three times more potent than oral ingestion, making it useful for postoperative and chronic pain treatment as well as for minor surgical procedures. In 1898, heroin was introduced commercially. Initially, it was thought to be less prone to abuse than morphine, and was marketed as a “morphine step-down cure.” Soon, however, it became apparent that the withdrawal symptoms were equally severe.
In the late 1960s, the concept of multiple opioid receptors was hypothesized, in part because of the distinct behavioral syndromes produced in animal studies by the different classes of opiate drugs.
9 This group of investigators named the postulated opiate receptor types by the opiate drugs they used in their studies—morphine activated the mu (μ) receptor, ketocyclazocine activated the kappa (κ) receptor, and SKF-10,047 activated the sigma (σ) receptor.
9,
10 The mouse vas deferens bioassay provided the name for a fourth type, the delta (δ) receptor.
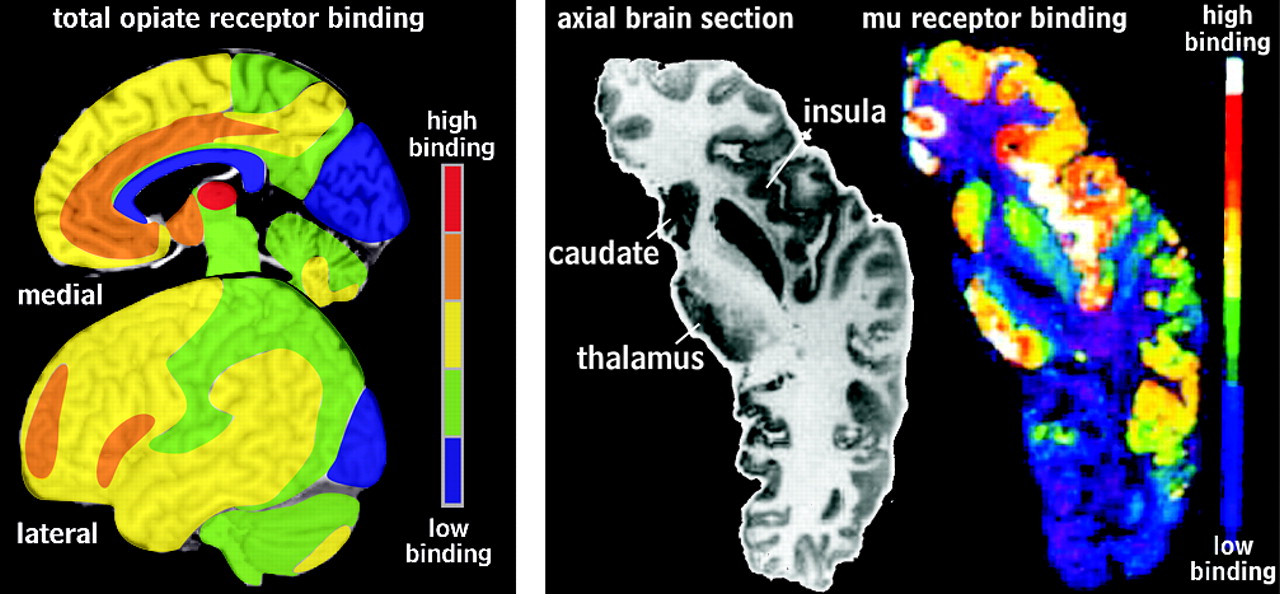
10Localizing the distribution of these receptors in the human brain has been the focus of much research. Early studies explored the differential regional distribution of these opiate receptors based on radioligand binding assays.
11 Total opiate binding was highest in the amygdala, insula, caudate, and anterior hypothalamus, followed by frontal and parietal cortex, putamen, thalamus, and periaqueductal gray in the midbrain. This technique requires homogenization of brain samples, and so provides limited spatial information. More recent autoradiographic and positron emission tomography (PET) studies provided much more detailed maps of opiate receptor localization (
Figure 1 ).
1 –
4 Mu receptors are plentiful in the periaqueductal midbrain, amygdala, thalamus, striatum, all areas of the cortex (most densely in layers I, III, and IV) except medial occipital and are present to a lesser extent in the cerebellum (granular layer).
12 –
15 Kappa receptors are most prominent in the cerebral cortex (most densely in layers V and VI everywhere but the occipitotemporal cortex, where receptors are concentrated also in the superficial layers), amygdala, basal ganglia, hippocampal complex, and cerebellum (molecular layer).
12 –
16 Delta receptors are more sparse in the human brain, in contrast to other species. They are most concentrated in the cerebral cortex (most dense in the superficial layers) and striatum, with lower levels in the hippocampus and amygdala. Binding is lower still in the thalamus and absent in the cerebellum.
12 –
15,
17 Thus, opiate receptors are found principally in limbic and limbic-related areas of the brain. The opiate system plays a role in many behaviorally important functions, including reward and reinforcement, modulation of the response to pain and stress, as well as homeostatic regulation.
18 Stimulation of mu receptors produces analgesia, euphoria, and miosis, as well as reinforces “reward” behavior. Stimulation of kappa receptors produces the subjective sensation of dysphoria, spinal anesthesia, sedation, and miosis.
19,
20 Delta receptors, like mu receptors, are involved in analgesia, though they have been less extensively studied.
The recent development of receptor-specific PET ligands has made possible the investigation of dynamic changes in opiate receptor function.
21 Carfentanil citrate is the most frequently used ligand for the imaging of the mu receptor. Recently, a stereospecific kappa ligand with good in vitro characteristics was labeled “GR 103545.”
21,
22 Delta receptors have been localized by the use of naltrindole, a potent and highly selective delta receptor antagonist.
21,
23,
24Opiates are highly addictive and, therefore, have the potential to be abused. Most opiates are available in pill form and are taken orally. Heroin can be swallowed, sniffed, or dissolved in water and injected intravenously (“mainlining”) or subcutaneously (“skin popping”). Fumes from the heated powder are inhaled (“chasing the dragon”). Some users combine opiates with a stimulant such as cocaine (“speed balling”). The newest opiate is a potent oxycodone variant. Designed for sustained release, it contains up to 20 times the normal amount of the active ingredient. Crushing the pills destroys their slow-release polymer. The resulting powder can then be swallowed, snorted, or injected, yielding a heroin-like high.
8Like other forms of addiction, opiate abuse can be a chronic relapsing/remitting disorder. The devastating lifelong effects of heroin addiction were recently highlighted in a report of a 33-year prospective study.
25 A cohort of male heroin addicts admitted to the California Civil Addict Program (a compulsory drug treatment program for criminal offenders) during the years 1962 through 1964 were interviewed at 10-year intervals. Of the original 581 subjects, 48.9% had died by the third time point (21.6% drug overdose, 19.5% homicide, suicide, or accident). Of the surviving 242 subjects, 20.3% had been incarcerated during the year prior to the interview, 13.7% had engaged in drug dealing, and 7.4% in property crimes. Approximately one-half had been abstinent for 5 or more years. Relapse was common (25%) even after 15 years of abstinence, emphasizing its chronicity.
A variety of neuropathological changes have been found in persons with a history of opiate abuse; there is no specific change that is characteristic.
26,
27 Rarely, a toxic spongiform encephalopathy can result from inhalation of heroin pyrolysate vapors (chasing the dragon). It was first described following an outbreak among heroin smokers in the Netherlands.
28 Clinically, three stages have been described.
28,
29 Stage 1 is characterized by predominantly cerebellar findings, including dysarthria, ataxia, motor restlessness, and bradyphrenia. Stage 2 includes pyramidal signs, pseudobulbar reflexes, spastic paresis, tremor, chorea, myoclonic jerks, and choreoathetoid movements. In stage 3, symptoms progress to stretching spasms, hypotonic paresis, akinetic mutism, central pyrexia, and death in approximately 25% of patients. Neuroimaging has been remarkably similar in most cases. The most characteristic finding is selective involvement of the posterior limb of the internal capsule and sparing of subcortical U fibers. Magnetic resonance imaging (MRI) typically reveals diffuse symmetric bihemispheric lesions of the cerebellum and the occipital, parietal, and temporal white matter. There is relative frontal sparing as well as involvement of the corticospinal tracts. These findings are best appreciated on fluid attenuated inversion recovery (FLAIR) sequences.
30,
31 Histological findings typically reveal spongiform degeneration of the deep white matter with vacuolization of the myelin sheath. Axons are usually well preserved despite the changes in myelin.
29,
30 The rare occurrence of this condition indicates that it is likely due to adulterants rather than to heroin itself, although the etiology is poorly understood. There have been cases reported in which the brainstem and cerebellar white matter were spared and axons were injured, suggesting the presence of a different impurity.
32 A defect in mitochondrial function has been suggested, as well as hypoxic-ischemic conditions, based on magnetic resonance spectroscopy findings.
30,
33 Supportive care and antioxidant therapy have resulted in favorable outcomes in some cases.
30,
34One study
35 has reported gray matter, but not white matter, reductions in prefrontal, insular, and temporal cortices in patients on methadone maintenance compared with age- and sex-matched healthy comparison subjects. The results were the same when the groups were divided by age (<39 years versus >39 years), but the effect of duration of opiate use was not specifically addressed. Two studies have reported lower than normal concentration of
N -acetylaspartate, as measured by in vivo magnetic resonance spectroscopy, in frontal gray matter of patients maintained on methadone, buprenorphine, or heroin.
6,
36 N-acetylaspartate is considered to be a marker of neuronal viability. These studies do not allow differentiation of preexisting conditions from possible detrimental effects of opiate abuse.
Several studies have reported patchy decreased regional cerebral blood flow (rCBF) as measured by single photon emission tomography (SPECT) in opiate-dependent subjects (methadone-maintained or heroin using).
37 –
39 These were most often located in the frontal and temporal cortex but were also found in other areas. Focal areas of increased rCBF were also present in some individuals. One study
38 reported no differences in rCBF related to type of opiate used (methadone, heroin, morphine). There was, however, a significant correlation between duration of use (>48 months) and reduced rCBF in mesiofrontal cortex bilaterally.
38 Overall, these results indicate that opiate abuse, like many other forms of drug abuse, is associated with alterations in rCBF. The reported differences in most affected cortical areas suggest that these changes may be highly individualized. A similar pattern of patchy decreased rCBF has also been reported during the first week of detoxification.
40,
41 One study reported improved rCBF in patients rescanned at 3 weeks of withdrawal, particularly in the frontal cortex.
40 Another study
39 found no improvement in a single subject rescanned at 10 weeks of detoxification. Though most of these studies included a psychiatric interview and excluded subjects with other axis I diagnoses, the presence of subclinical psychiatric states that might affect rCBF cannot be ruled out.
In contrast to rCBF findings, one study measured the regional cerebral metabolic rate (rCMR) by fluorodeoxyglucose PET (FDG-PET) and found that rCMR in the anterior cingulate cortex was significantly elevated in abstinent opiate-dependent subjects.
42 The subjects had a minimum of 6 months since detoxification from methadone; they were compared to nondrug-using individuals. Although differences in other brain regions were not statistically significant, the trend was for rCMR to be highest in the abstinent opiate-dependent group and intermediate in the methadone-maintained group compared to the nondrug-using group. The authors of this study noted that these results suggest that chronic opiate use may result in long-lasting neurobiological abnormalities.
Previous research has provided evidence of performance impairment and impulsivity in substance abusers. In particular, performance may be impaired on cognitive tasks that require decision-making that involves balancing short-term rewards and long-term consequences.
43 –
46 Multiple recent studies attempt to define more specifically areas of impairment in abstinent or medically maintained opioid-dependent patients. This research is of great value as the efficacy of methadone maintenance for opioid-dependent persons has long been debated. A Cochran review noted that “Methadone is an effective maintenance therapy intervention for the treatment of heroin dependence as it retains patients in treatment and decreases heroin use better than treatments that do not utilize opioid replacement therapy. It does not show statistically significant superior effect on criminal activity.”
47 Thus, it is necessary to explore the clinical significance of cognitive impairment on daily performance and ability to control behavior.
Studies have compared performance of methadone-maintained patients to healthy subjects on a range of cognitive and psychomotor tasks.
48 –
55 Reported differences include impairment in psychomotor/cognitive speed, attention, working memory, and decision-making. However, results on particular tasks differ considerably across studies. One source of variability may be the degree of matching between the methadone-maintained patients and comparison groups on demographics (e.g., age, years of education, employment status) and comorbid disorders.
48,
50,
56 Another potentially important factor may be duration of methadone treatment, as a recent prospective longitudinal study has reported that some measures of cognitive performance improved between 2 weeks and 2 months of methadone maintenance.
57 Another possible source of variability may be in the level of reinforcement a task provides for individuals from these various comparison groups. A recent study found that methadone-maintained subjects rated the subjective value of a monetary reinforcement lower than the healthy comparison group.
58 Differential exposure to conditions likely to result in acquired brain injury (e.g., head trauma, nonfatal overdose) may also contribute to variability in impairment.
49 If specific impairments differ considerably among individuals within a group, as the differences among these studies suggest, the more global approach of defining “cognitive impairment” as two or more clearly abnormal test scores (e.g., 2 standard deviations from the mean) may be useful.
52Of particular interest is whether cognitive performance improves with abstinence. Several studies have compared the methadone-maintained patients to former heroin users on a variety of tasks, with mixed results.
52,
53,
55,
59,
60 One laboratory found both groups similarly impaired on measures of executive function, visual memory, and impulsivity.
55,
61 Although neither group was impaired on the Cambridge Risk Task, a measure of risky decision-making, only methadonemaintained patients were more likely to make a higher risk choice immediately following a loss.
62,
63 Three studies found impaired performance in the methadonemaintained patients compared to the abstinent group. In one study, the groups differed only on verbal fluency after controlling for age.
52 Another study found more extensive differences, including impaired processing speed, response inhibition, visuospatial attention, and cognitive flexibility in the methadone-maintained group.
60 The third study reported that the abstinent group performed significantly better than the methadone-maintained patients only on cognitive flexibility and set shifting, and was intermediate between methadone-maintained patients and healthy subjects on most measures.
59 In contrast, another study reported that the only difference between the groups was more impaired visuospatial memory in the abstinent group.
53 Overall, these results do not strongly indicate recovery of function with abstinence. However, one study also compared the groups using the more global measure of “cognitive impairment” (as described above).
52 More of the methadone-maintained patients were cognitively impaired (9/15, 60%) than of the abstinent group (5/16, 31%). The authors of this study noted that this marginally lower incidence of cognitive impairment may indicate some recovery of function, but it is very likely due more to lifestyle changes than the absence of methadone.
An alternative interpretation of studies indicating greater impairment in methadone-maintained patients than abstinent groups is that methadone itself may contribute to cognitive impairment. A recent study of methadone-maintained patients found that administration of methadone in a single daily dose (rather than divided into a morning and an evening dose) impaired episodic memory.
64 The authors of this study noted the importance of episodic memory to both day-to-day functioning and success for therapeutic interventions. Buprenorphine is also used for maintenance treatment of former opioid abusers. It is a partial mu receptor agonist and kappa receptor antagonist, unlike methadone, a pure mu agonist. There is evidence that buprenorphine may cause less psychomotor and cognitive impairment that methadone.
65 Several recent prospective studies have directly compared the effects of methadone versus buprenorphine maintenance.
54,
66,
67 In one study, baseline neuropsychological measures were obtained from both groups of patients during the first week of treatment to assure comparability in performance on tests of attention, memory, and verbal fluency.
66 Although the differences were not large, the buprenorphine-maintained group tended to have better psychomotor performance at 8 to 10 weeks of treatment. Two studies
54,
67 have included some measures of decision-making. In both studies, the methadone-maintained group was impaired, whereas the buprenorphinemaintained group performed similarly to healthy subjects.
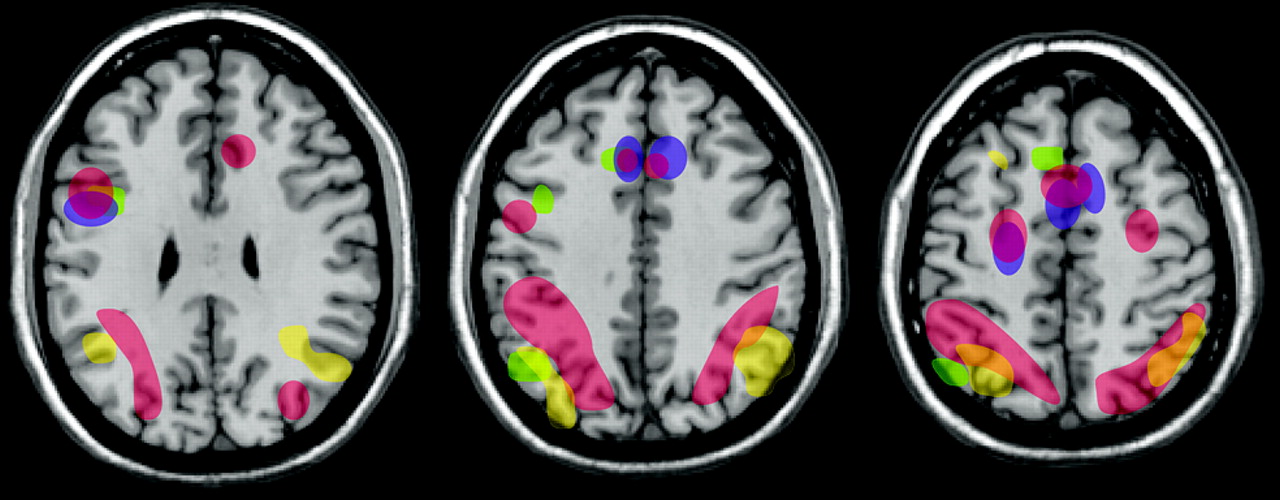
Poor behavioral regulation may stand at the core of substance abuse, and understanding its neural basis could provide important advances in the treatment of addiction. Several recent studies have compared error-related brain activation patterns between opiate users and healthy subjects during tasks requiring behavioral regulation (e.g., go/no go) that activate the anterior cingulate cortex in normal healthy individuals.
5,
6,
68 All three studies found absent or diminished error-related activation in the anterior cingulate cortex in opiate users. The two that also surveyed other areas of the brain found increased activation in parietal and temporal areas, suggesting compensatory recruitment in order to perform the task (
Figure 2 ). As noted by many researchers, differentiating preexisting traits or findings from effects of drug dependence is often very challenging. Determining whether differences in brain morphometry, task-related brain activation, and behavioral regulation are predisposing conditions that increase the risk of dependence is critical to understanding the devastating effects of addiction. Study of other addictive conditions that do not involve substance abuse, such as pathological gambling, may help to resolve this important question.