D enial of illness is frequently found among stroke victims. These patients may be less likely to seek treatment and more likely to engage in potentially dangerous activities, leading to poor outcomes. Denial usually develops following right hemisphere stroke, but not exclusively, and its etiology is unknown. We have previously reported that approximately one third of acute stroke patients developed denial, and suggested that frontal-lobe dysfunction might play an important role.
1 We also developed the Denial of Illness Scale and demonstrated its reliability and validity.
1 The neural basis for executive function has been extensively studied. It has been shown that frontal-subcortical circuits form five parallel networks.
2 They are the motor circuit, oculomotor circuit, dorsolateral prefrontal circuit, lateral orbito-frontal circuit, and anterior cingulate circuit. Among them, the last three circuits are believed to be important in executive function, especially the dorsolateral prefrontal circuit.
3 A variety of stroke lesions could impair executive function via these circuits.
Case Example
The following brief case discussion, which describes a participant in the current sample, is included here to provide an example of what is meant by the term “denial of illness.”
Mr. L, a 71-year-old, right handed, married, retired Caucasian male, suffered a sudden onset of left facial weakness and ataxia. On examination, he showed left central 7th nerve palsy, left visual field defect, left hemi neglect, left sided muscle weakness, and clumsy ambulation. He also had mild dysphagia. He was fully alert and oriented, but somewhat impulsive, and developed mild cognitive dysfunction including difficulty with simple calculation, problem solving, memory, and organization. A brain CT revealed a right middle cerebral artery territory ischemic infarction, especially the right parietal region. Despite having the above symptoms, the patient indicated that nothing was wrong with him, he denied having any paralysis or weakness, and he noted that he was feeling very good and was ready to go home. He was finally able to show some acknowledgment of his symptoms after they were pointed out and explained to him during a neurological exam. Despite this, he ate impulsively, neglecting instruction from his speech therapist, and finally developed aspiration pneumonia due to dysphagia. He frequently minimized his stroke related symptoms, was very cheerful, pleasant, and nonchalant, and did not show any concern about his physical condition.
METHOD
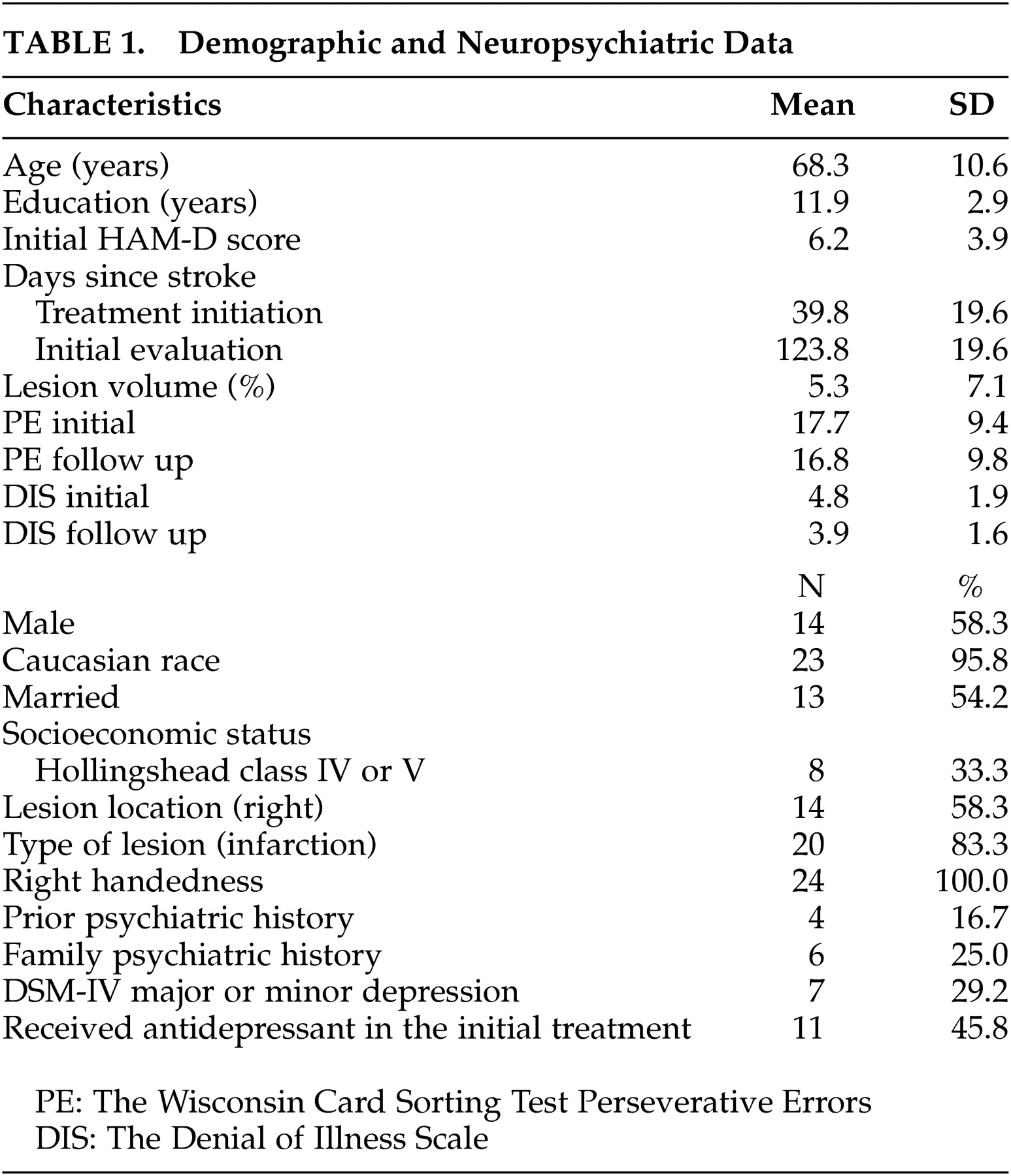
Subjects were consecutive admissions to Younkers Rehabilitation Center of Iowa Methodist Medical Center in Des Moines, Iowa, between June 1991 and June 1997. Patients were enrolled within 6 months following stroke. Exclusion criteria were significant medical illness, severe comprehension deficit, prior history of neurological disease, Mini-Mental State Examination (MMSE) score <23, and taking antidepressants. Written consent was obtained in accordance with our Institutional Review Board. After 12 weeks of treatment (either nortriptyline, 100 mg/day maximum, fluoxetine, 40 mg/day maximum, or placebo), 44 patients were assigned to a 21-month naturalistic follow up study. Among them, 7 declined any additional assessments, 7 patients refused further evaluation during the 21 months follow up, 4 developed physical complications, and 2 died. Only one patient in the drop-out group showed minimal denial of illness. Thus, 24 patients completed all of the evaluations. Background variables of withdrawn patients were not significantly different from the 24 patients who completed the assessment. The present study was conducted as a part of the original larger scale study.
6At the initial and the follow-up evaluations, the Denial of Illness Scale was given along with the 17-item Hamilton Depression Rating Scale (HAM-D), MMSE, the Wisconsin Card Sorting Test, Social Function Examination, Johns Hopkins Functional Inventory, and Social Ties Check List. Briefly, the Denial of Illness Scale is a 10-item, 20-point scale on which higher scores indicate greater levels of impairment, which was originally designed by Hackett and Cassem
7 and was later modified by our group for brief and practical clinical evaluation of denial of illness.
1 The Wisconsin Card Sorting Test requires the subject to form problem-solving strategies (the subject must determine various ways in which cards can be organized depending on the characteristics of the cards) and to alter these strategies in response to external feedback. As such, it is considered to be a measure of executive function. Among multiple variables yielded from the Wisconsin Card Sorting Test, the number of Perseverative Errors was used as a measure of conceptualization and problem-solving ability. The Johns Hopkins Functional Inventory is a 10-item scale that measures activities of daily living. It is a 44-point scale with higher scores indicating greater levels of impairment. The Social Functioning Exam is a 28-item scale that assesses patients’ satisfaction with their social functioning. Scores on the Social Functioning Exam range from 0.00 to 1.00, with high scores indicating greater severity of social impairment. The reliability of these instruments has also been previously demonstrated.
6 Computerized tomography or magnetic resonance scans were used to assess for lesion volume and location using standardized methodology.
6 All examinations were performed in a double-blind fashion.
The relationship between the Denial of Illness Scale and other measurements was examined using nonparametric correlation analysis (Spearman’s Rho). All statistical tests were two-tailed with an alpha level of .05.
RESULTS
Background information of the 24 patients is shown in
Table 1 . There were no significant differences between active and placebo treated groups in any variables including lesion volume and location. Thus, all the 24 patients were analyzed as a single group.
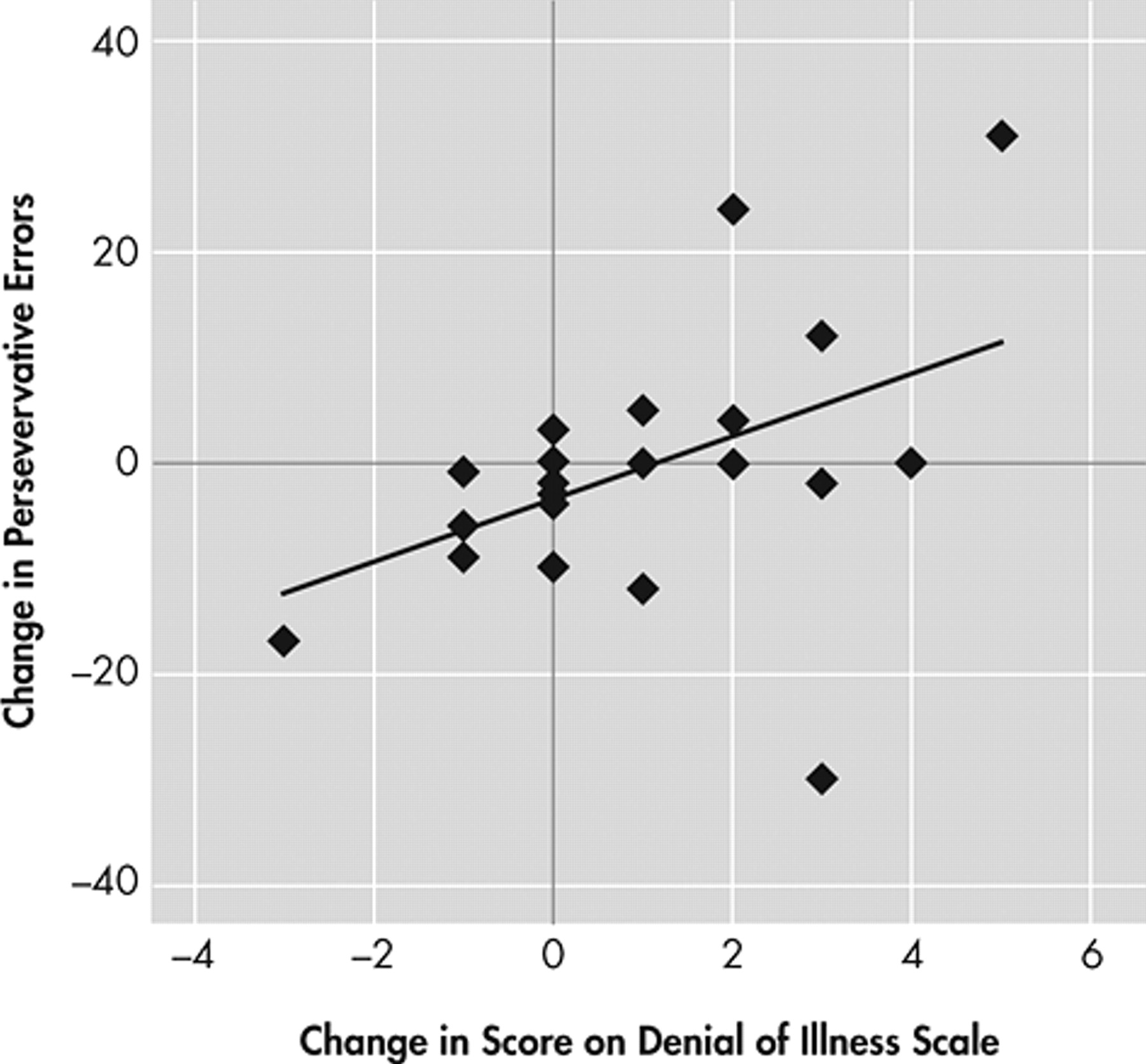
Changes between the initial and the follow-up evaluations were assessed using change scores. The correlation between longitudinal changes in denial of illness and executive function was evaluated as an association between change scores on the Denial of Illness Scale and the Wisconsin Card Sorting Test Perseverative Errors, which turned out to be moderate and statistically significant (Spearman’s r
s =0.49, p=0.02; removing one outlier who was >3 SDs from the mean on the Wisconsin Card Sorting Test, r
s =0.64, p<0.01) (all remaining analyses included the outlier) (
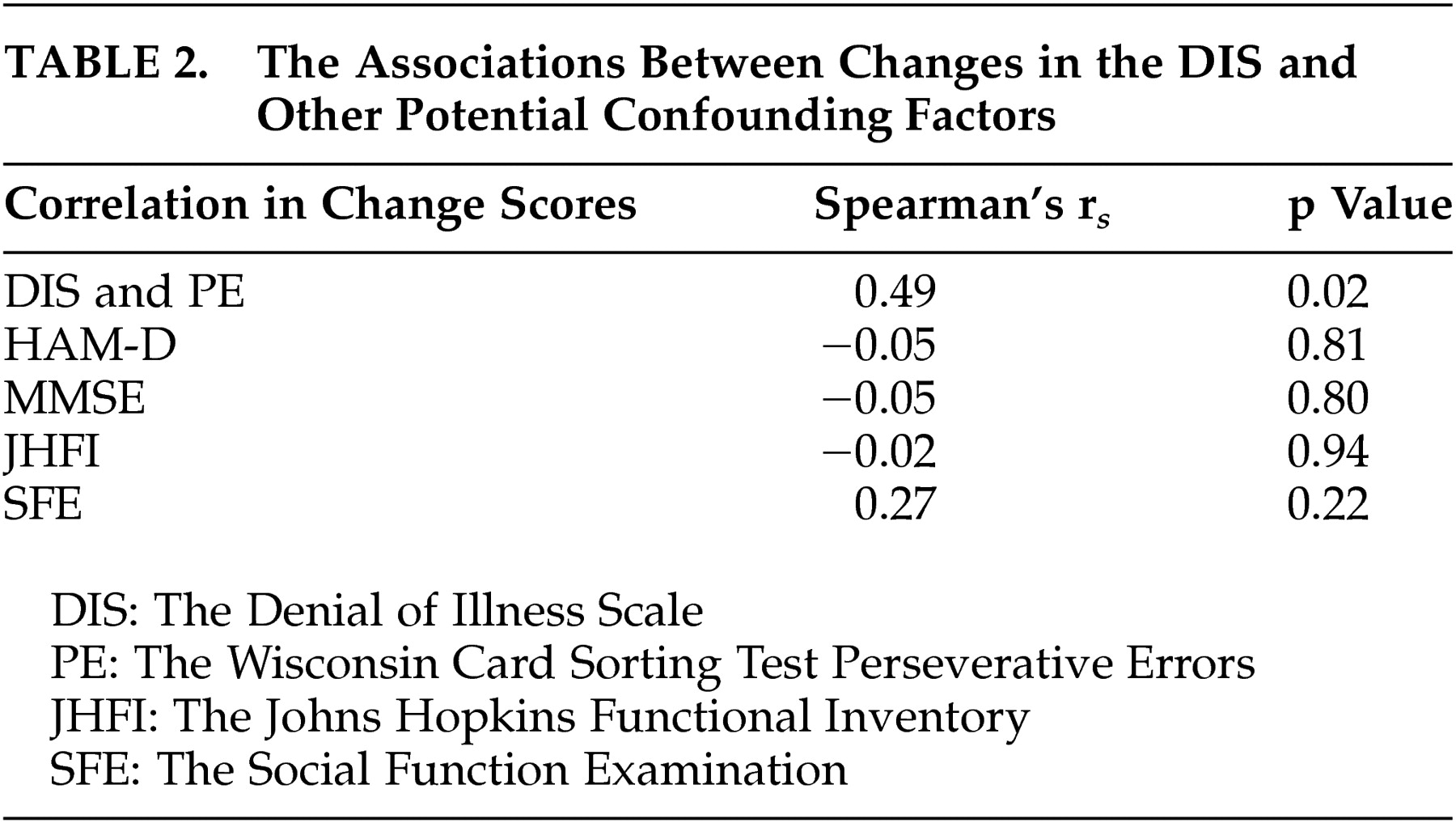
Figure 1 ). Among actively treated patients, the Rho was as high as 0.75 with p value less than 0.01. The associations between changes in the Denial of Illness Scale and other potential confounding factors noted above, such as HAM-D, were also evaluated. None of them was found to be significant (
Table 2 ). The small number of patients precluded us from performing covariate or multiple logistic regressions.
As a whole, there was no significant improvement between initial and follow-up evaluations in either the Denial of Illness Scale or the Wisconsin Card Sorting Test Perseverative Errors (
Table 1 ); however, the comparison of those who showed improvement in the Wisconsin Card Sorting Test and those who did not showed a significant difference in the Denial of Illness Scale change scores (F=5.37, df=1,22, p=0.03).
DISCUSSION
The correlation between changes in denial (the Denial of Illness Scale) and executive function (the Wisconsin Card Sorting Test Perseverative Errors) over 2 years following stroke was positive and significant in the absence of global cognitive dysfunction. The changes in other possible confounding factors including other cognitive, psychiatric, and neuroimaging measures, were not significantly associated with improvement in denial symptoms.
Before discussing these findings, some limitations should be acknowledged. First, the majority of patients in the study were high school or college-educated, Caucasian, married, and in a relatively high social class. Therefore, the results of this study may not be applicable to all poststroke patients. Second, we were not able to obtain follow-up evaluations in all of the patients, which may have influenced our findings. Third, the sample size was relatively small and this might have limited our statistical power to find other correlations. Fourth, we did not evaluate executive function and denial of illness in the acute phase after stroke and our evaluation was limited to specific aspects of executive function (i.e., conceptualization and problem-solving). Additionally, participants with MMSE <23 were excluded, which might have affected these findings. Despite these caveats, the above findings could shed some light on the investigation into the mechanism of denial of illness.
A significant and positive correlation was found between longitudinal changes in the Denial of Illness Scale and the Wisconsin Card Sorting Test Perseverative Errors. As shown in our previous study, antidepressant intervention during the acute phase following stroke improved longitudinal outcome of executive function.
5 In this study, it was shown that improvement in severity of denial was significantly and positively associated with improvement in executive function. This association was significant and stronger among actively treated patients but it was also true when patients given placebo were added. Other potential confounding measurements did not show significant correlation with improvement in denial symptoms.
How might these findings be construed? There have been many attempts to explain denial of illness or similar pathological phenomena. Briefly, Weinstein and Kahn
8 proposed a psychological defense mechanism as a possible etiology. Hecaen et al.
9 proposed that denial was the result of a confusional state due to medical illness. Levine
10 suggested that lack of sensory feedback to the brain would explain such phenomena, while some believed it was related to disconnection between hemispheres. Each of these hypotheses explained denial of illness to some extent, but not fully.
Denial is not specific to stroke; in fact, it can occur across multiple conditions. For example, “lack of awareness” or “poor insight” is frequently reported in schizophrenia,
11 Huntington’s disease,
12 Alzheimer’s disease,
13 fronto-temporal dementias,
14 metabolic syndromes such as Lesch-Nyhan syndrome,
15 and even normal pressure hydrocephalus.
16 It seems most plausible that the denial symptoms in the above conditions share some underlying etiology.
Patients with schizophrenia
11 are generally believed to have “hypofrontality.”
17 A recently published study reported that drug-naive schizophrenia patients with unawareness of illness showed significant volume loss in the right dorso-lateral prefrontal cortex.
18 In Huntington’s disease, compromised fronto-striatal integrity is frequently reported.
19 With Alzheimer’s disease, a decreased metabolism in orbital prefrontal cortex has been shown.
20 Fronto-temporal dementia patients have impaired frontal lobe function, and in normal pressure hydrocephalus, a regional reduction in cerebral blood flow in the frontal-temporal cortical regions has been recognized.
21 Thus, it would be reasonable to say that denial is frontally mediated and to hypothesize that it would be associated with executive dysfunction. In the present study, it was shown that severity of denial was significantly related to executive dysfunction, specifically conceptualization and problem-solving ability. Denial seems to be a general “biological” condition due to deterioration in specific domains of executive function rather than a disease-specific phenomenon.
As noted, no significant correlation was found between changes in the Denial of Illness Scale and Johns Hopkins Functional Inventory or the Social Functioning Exam (
Table 2 ), which means that improvement in denial does not necessarily imply better ADL (Activities of Daily Living) functioning. The reason for this is unclear. Perhaps there is a time lag between improvement in executive function and actual improvement in daily activities. Additionally, as shown in the literature,
22,
23 denial of physical impairment does not imply denial of emotional disorder. This may explain the discrepancy noted above, at least to some extent. Further studies are needed to clarify these fundamental issues.
In summary, our data show that denial symptoms are significantly associated with executive function in longitudinal outcome following stroke, regardless of antidepressant intervention in the acute phase. This suggests a role for executive dysfunction in the mechanism of denial of illness.
Acknowledgments
The authors would like to thank Stephan Arndt, Ph.D., Teresa Kopel, M.A., Stephanie Rosazza, B.S., J. Todd Kosier, B.S., as well as the Younkers Rehabilitation Center of the Iowa Methodist Medical Center in Des Moines, Iowa, for allowing the authors to assess patients for inclusion in the study. This work was supported in part by NIMH R01 grants MH-40355, MH-52879, MH-53592, MH63405, and Research Scientist Award MH-00163 (RGR).