A pathy—a lack of motivation, interest, or initiation—has received much attention in neuropsychiatry. In this context, apathy has been conceptualized as the behavioral and cognitive result of a neurological lesion (such as a frontal or frontal-subcortical lesion). Cross-sectional studies have found clinically significant apathy symptoms in one-quarter to one-half of elderly patients with neurological illnesses such as Alzheimer disease,
1 –
3 extrapyramidal diseases,
4 –
5 and stroke,
6 compared with a prevalence of 1%–2% in the elderly community.
7 As reviewed elsewhere, studies have found that apathy in neuropsychiatric disease is associated with greater functional impairment, depressive symptomatology, and cognitive dysfunction.
8 –
9 A frequent clinical debate of “is it depression or is it apathy?” has the goal of contrasting depression, a treatable condition, with apathy, conceptualized as a fixed deficit.
4,
9 –
11 However, apathy could also arise after a severe stressor. Disabling medical events, such as a hip fracture, produce not only psychological stress but also immobility and pain. The range of reactions to such stress includes a decrease in goal-directed behavior as well as dysphoria.
12Disabling medical events, then, could lead to apathy syndromes in elderly persons in the same way that depression frequently develops in this context.
13 Apathy is a potentially critical variable in this population, which faces intensive rehabilitation and other challenges to recovery, as it predicts poorer rehabilitation participation and poorer functional recovery in geriatric rehabilitation.
14 If apathy in this context is subject to change, it could be a target for treatment. However, no research has examined
changes in apathy after a disabling medical event.
In the current study, we used the Apathy Evaluation Scale,
15 an interview-based measure of apathy symptoms and signs, to assess apathy prospectively in elderly hip fracture patients. We hypothesized that apathy symptoms would predict functional recovery, and that those with initially high but improved apathy symptoms would have better functional recovery than those with persistently elevated apathy.
METHODS
Sample Recruitment
The study recruited consecutive hip fracture admissions to an acute care hospital from March 2002 to October 2004, as described previously.
16 Participants received surgical repair and were ages 60 or older, able to provide informed consent, free of metastatic cancer, and had a Mini-Mental State Examination (MMSE)
17 score greater than 15. This study was approved by the university’s institutional review board.
Measures
Participants were prospectively observed for 6 months. They were assessed at the end of their acute hospital stay (i.e., at baseline) and then 2 weeks later with the Apathy Evaluation Scale (AES)
15 to measure signs and symptoms of apathy. The AES is a comprehensive measure of the observable behavioral, cognitive, and emotional concomitants of goal-directed behavior; it has 18 items and scores range from 18 to 72, with higher scores indicating more apathy. It measures how a subject feels at a particular time (rather than functioning as a dispositional or trait-like measure) and therefore can be used to examine changes in apathy over time. Cronbach alpha for the measure in this study was 0.93. Participants were also assessed for depressive symptoms with the 17-item Hamilton Depression Rating Scale (HAM-D)
18 and the Cornell Scale for Depression in Dementia.
19 –
20 Cognitive functioning was measured with the MMSE, the initiation/perseveration subscale of the Dementia Rating Scale,
21 the Trail Making test parts A and B,
22 and the logical memory subtest of the Wechsler Memory Scale, 3rd ed.
23 Delirium was measured with the Delirium Rating Scale.
24 Additionally, medical comorbidity was measured using the Cumulative Illness Rating Scale for Geriatrics.
25 This scale has items (scored from 0 to 4) for different domains of medical illness; for example, those with neurological illness (e.g., prior stroke) would have a positive score on the neurological item (#12).
Functional status was measured at baseline and at 2, 12, and 26 weeks later, using the 13 motor items of the Functional Independence Measure (FIM-motor).
26 This instrument rates dependency on other persons or assistive devices for activities of daily living and mobility tasks and is scored on a range from 13 (complete dependency for all activities of daily living and mobility tasks) to 91 (complete independence). For those who received rehabilitation in a skilled nursing facility or inpatient rehabilitation hospital, we measured their participation in physical and occupational therapy using the Pittsburgh Rehabilitation Participation Scale (PRPS),
27 a validated scale of patient participation in inpatient rehabilitation which provides a clinician-measured summary score (1–6, with higher scores indicating better participation) for each therapy session.
Analysis
The first hypothesis was that baseline apathy symptoms predicted functional recovery. Thus we examined (1) distribution of baseline AES scores and rate of clinically significant apathy symptoms, (2) demographic and clinical characteristics associated with apathy symptoms using Pearson correlation coefficients, and (3) baseline apathy symptoms as a predictor of trajectory of FIM-motor scores over 6 months, using a mixed effect repeated measures analysis.
Our second hypothesis was that improved apathy was associated with improved functional recovery. Thus we (1) examined distribution of changes from baseline to week 2 among initially high-apathy participants; (2) compared functional recovery between patients grouped by high versus low baseline and follow-up levels of apathy symptoms with persistently high AES scores (“high-high”) and persistently low-apathy patients using a mixed effect repeated measures model; and (3) examined correlates of improved apathy among cognition, depression, and other variables using Pearson correlation coefficients and t tests.
RESULTS
Study Group
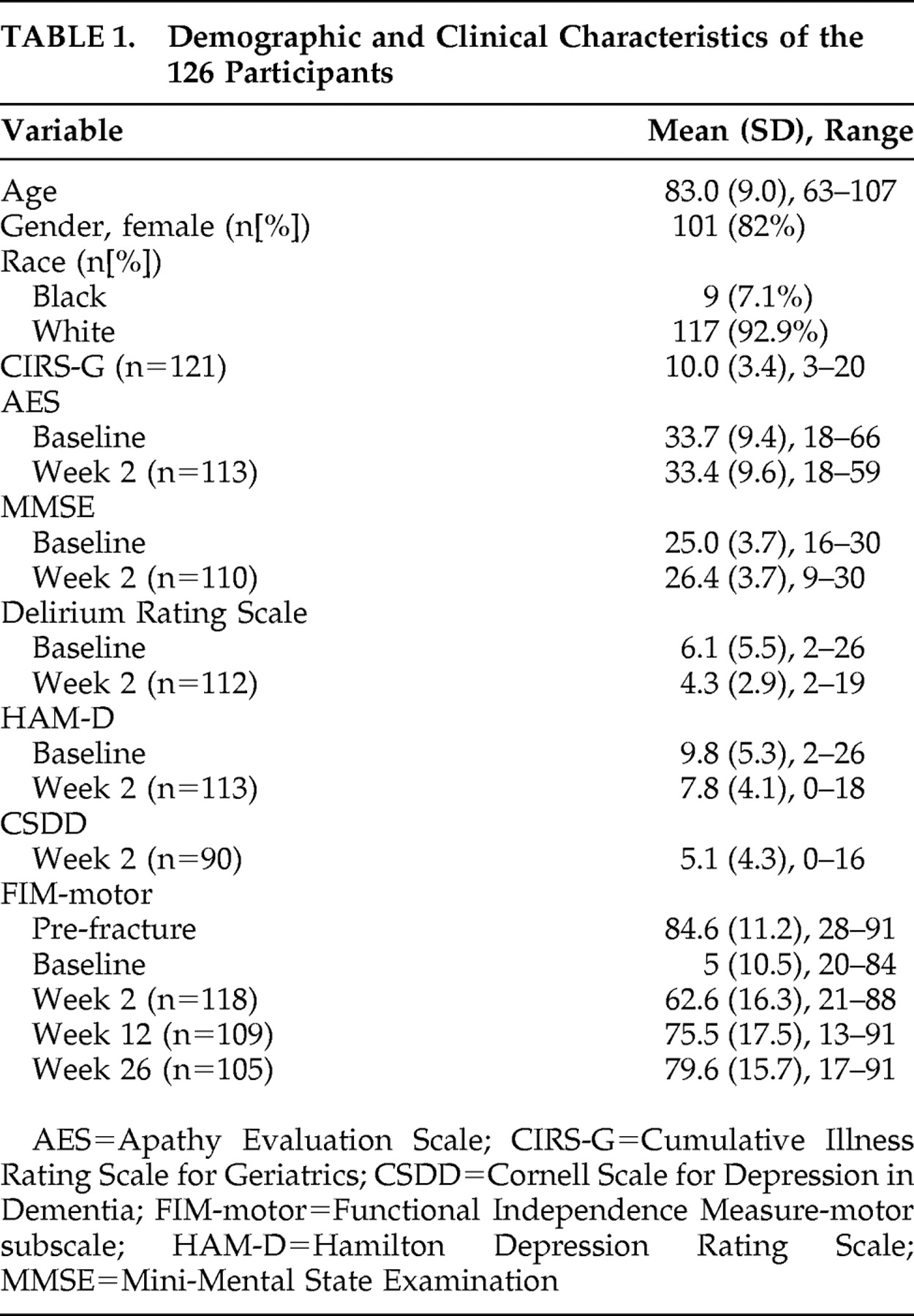
We approached 141 patients; 13 refused the study (or dropped out before providing baseline data) and two were excluded from further participation because of moderate to severe cognitive impairment. Thus, the study group comprised 126 patients. Baseline and week 2 characteristics of the participants are summarized in
Table 1 .
Rates of Clinically Significant Apathy Symptoms
The mean AES score was 33.7 (SD=9.4) at baseline and 33.4 (SD=9.6) at week 2. Examining the distributions found no natural cutoff; therefore, we chose the cutoff of ≥38 as a definition of clinically significant apathy symptoms, as previously reported.
15 By this cutoff, 46/126 patients (37%) had clinically significant apathy at baseline and 36/113 patients (32%) had clinically significant apathy at week 2.
Correlates of Apathy Symptoms
Next we examined the correlation of baseline Apathy Evaluation Scale (AES) scores with age and baseline clinical characteristics. Of the two depression measures, AES scores were significantly correlated (p<0.001) with both the Cornell Scale for Depression in Dementia (r=0.42, n=90) and HAM-D scores (r=0.40, n=126). Among measures of cognitive function, AES scores were significantly correlated (p<0.001) with general cognitive ability as based on the MMSE (r=−0.42, n=126) as well as measures of executive function (Delirium Rating Scale initiation/perseveration subscale, r=−0.57, n=82 and Trails B, r=0.51, n=88) and attention/psychomotor speed (Trails A, r=0.49, n=92), but not delayed recall (Logical Memory Test, r=−0.12, n=108). Scores on the AES scores also correlated (p<0.001) with Delirium Rating Scale scores (r=0.37, n=126), postfracture FIM-motor scores (r=−0.38, n=126), and age (r=0.27, n=126). Scores on the AES were not significantly associated with overall medical burden (Cumulative Illness Rating Scale for Geriatrics score, r=0.14, n=121) or neurological illness (Cumulative Illness Rating Scale for Geriatrics item 12, r=0.14, n=125). At baseline, 41 patients (33%) were taking sedative medications, and 64 patients (52%) were taking opiate pain medications; apathy scores were not different in those taking these medications versus those without, nor were these medications associated with functional recovery (data not shown).
For depression and delirium measures we examined correlations with and without items in those scales that overlapped with the apathy construct, consistent with prior literature.
6 Thus, we removed three items from the HAM-D (item 7, “work and activities,” item 8, “psychomotor retardation,” and item 13, “somatic symptoms general”), four items (items 3, 6, 8, and 11) from the Cornell Scale for Depression in Dementia, and one item (item 9, “apathy”) from the Delirium Rating Scale. However, removing these items did not appreciably change either the magnitude or significance of the correlations of AES with these measures (data not shown).
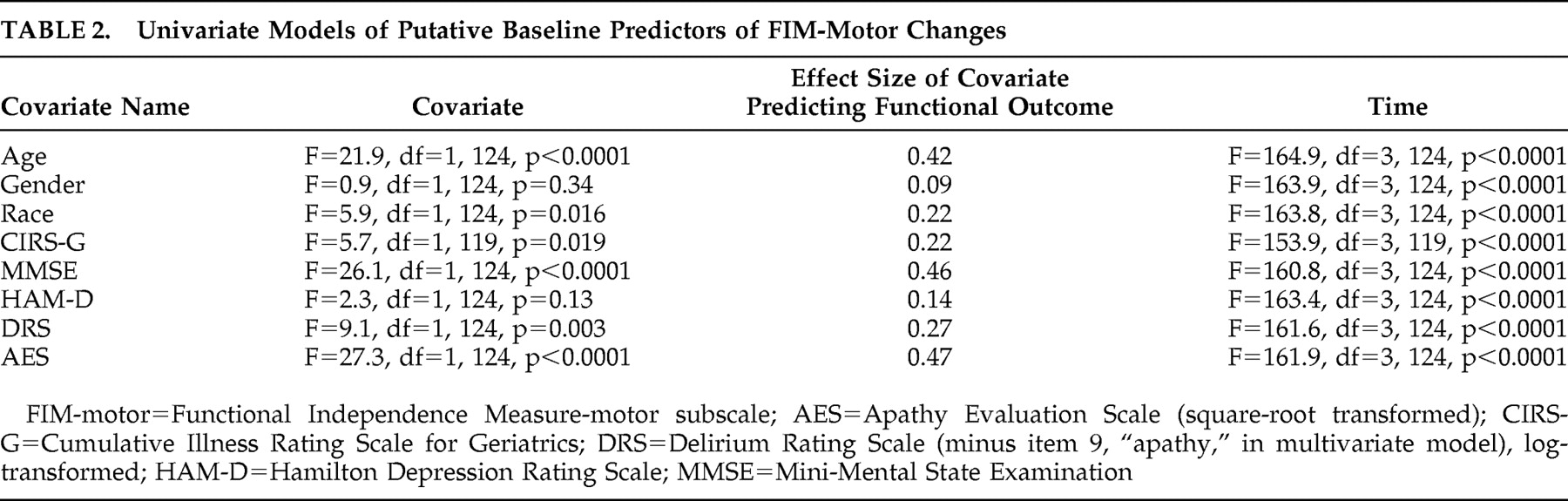
Hypothesis 1: Apathy Symptoms Predict Functional Recovery
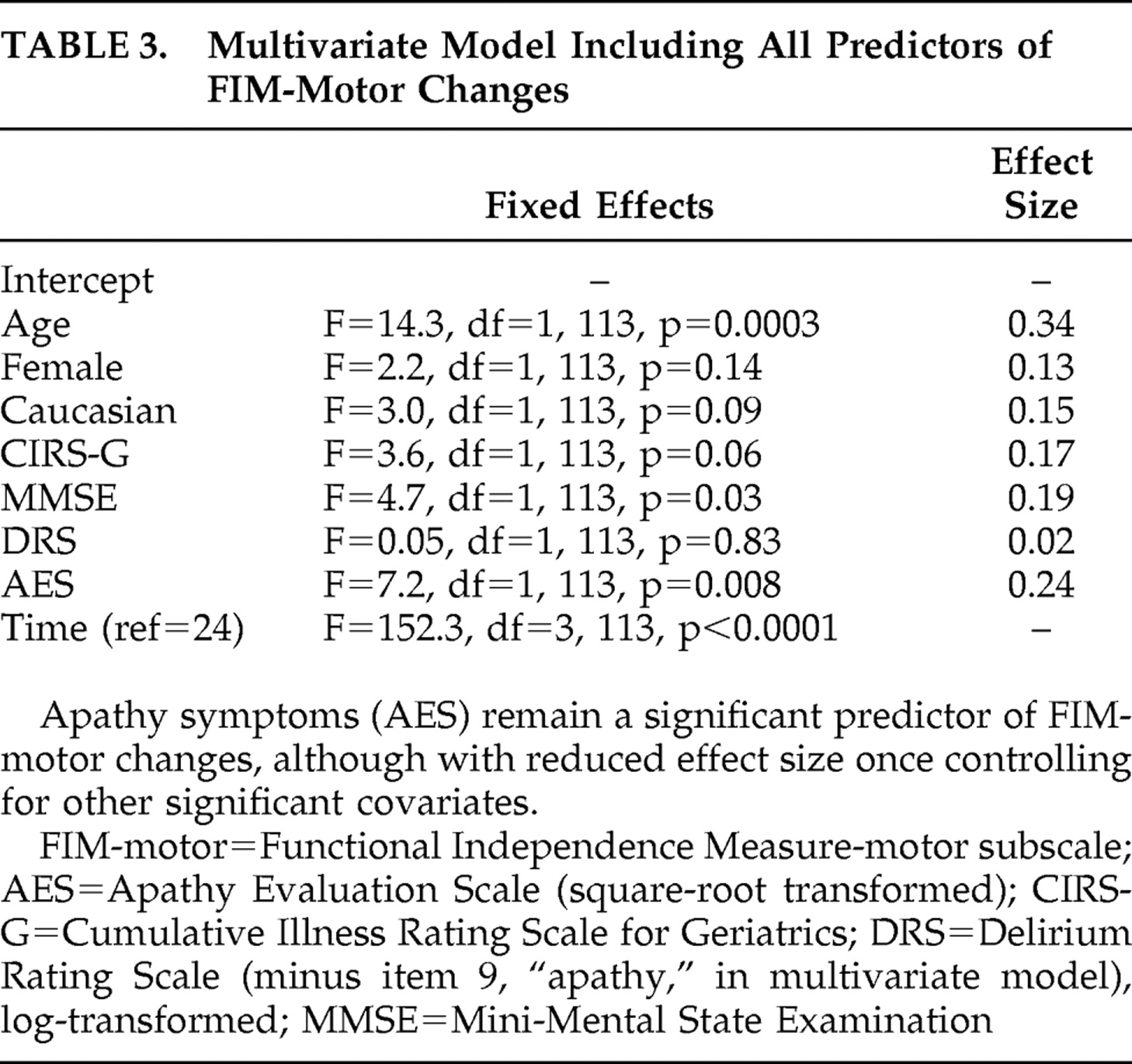
Table 2 shows the results of a series of univariate mixed-effects models predicting functional recovery from the baseline (immediate postfracture) period to 26 weeks. AES scores predicted recovery, with a moderate effect size, while depression measures were not significant predictors. In a multivariate model including all covariates that were potential predictors of recovery (
Table 3 ), AES scores remained a significant predictor of functional recovery, albeit with reduced effect size. Comparing high baseline apathy (AES >38) with low apathy groups in a mixed effects model found similar results; apathy group was a significant predictor (F=20.7, df=1, 124, p<0.001), with FIM-motor improvement at 2 weeks of 8.9 versus 27.6 in the high versus low groups, respectively. By 26 weeks, the FIM-motor improvement was 17.7 versus 31.4 in the high versus low groups, respectively.
Because these findings supported our hypothesis that baseline apathy symptoms predicted functional recovery, we then examined whether rehabilitation participation may account for the association of AES scores with functional recovery, as prior research has found that apathy predicts participation in rehabilitation
14 and that rehabilitation participation predicts functional outcome.
28 First, we confirmed that AES scores correlated with Pittsburgh Rehabilitation Participation Scale (PRPS) scores: baseline AES was correlated with mean PRPS scores in occupational therapy (r=−0.39, p<0.001, n=97) and physical therapy (r=−0.29, p<0.01, n=103). Next, we added mean PRPS scores to the multivariate model presented in
Table 3 . While PRPS scores predicted poorer functional outcome in this revised model (F=8.96, df=1, 96, p=0.0035, effect size=0.27), the effect size of the association of AES with functional outcome did not change. Finally, we examined a reduced model that included AES, PRPS, and an AES × PRPS interaction term; this term was trend-level significant (F=3.2, df=1, 104, p=0.08) while AES was not significant (F=0.6, df=1, 104, p=0.46). Ensuing correlations showed a significant relationship between PRPS and FIM-motor outcome in individuals with AES score ≥38, and no relationship among those with AES score <38.
Changes in Apathy
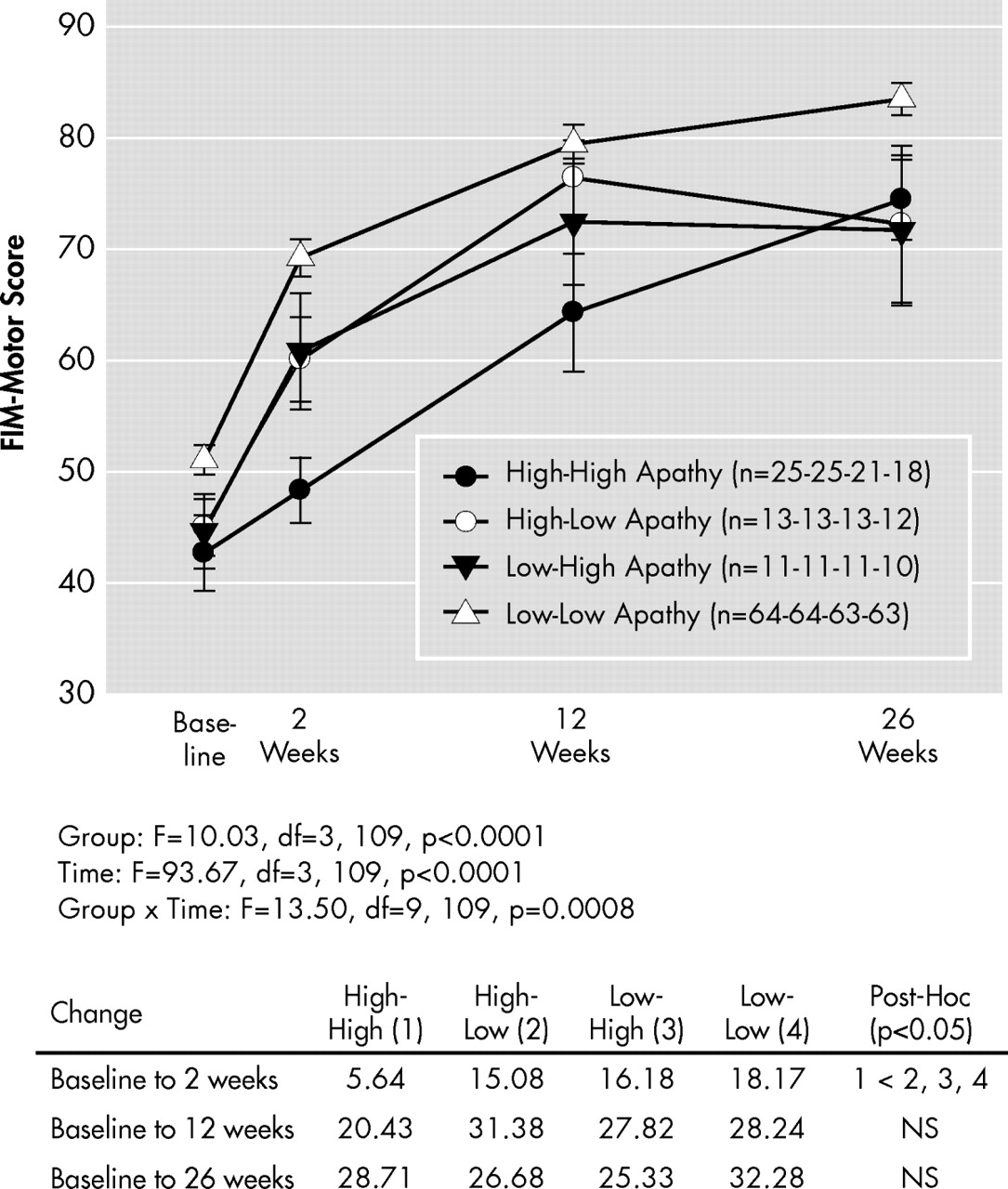
To examine changes in apathy from baseline to week 2, we divided the sample into four groups: those with high AES scores (≥38) at both time points (“high-high” or persistently high apathy, n=25), those with high baseline AES scores but with lower scores (<38) at week 2 (“high-low” or improved apathy, n=13), those with low AES scores (<38) at both time points (“low-low” or persistently low apathy, n=64), and those with initially low AES scores but high scores at week 2 (“low-high,” n=11). We grouped participants in this way to provide clinically salient observations. The improved apathy group had a mean (SD) change of −8.2 (4.5) in AES scores, corresponding to a 19% decline, from baseline to week 2, while the persistent apathy group had a change of 1.6 (5.7), corresponding to a 4% increase in scores.
Hypothesis 2: Improved Apathy is Associated With Better Functional Outcome Than Persistent Apathy
Figure 1 shows the trajectory of FIM-motor scores in these four groups. The main interest was to contrast those with improved (“high-low”) apathy with those with persistent (“high-high”) apathy. As the figure shows, individuals with improved apathy had a better functional recovery; however, the modeled data are suggestive of an effect early but not late in recovery. Therefore we ran post-hoc tests comparing the groups in terms of FIM-motor improvements at 2 weeks, 12 weeks, and 26 weeks. As this figure shows, those with initially high but improved apathy symptoms showed significantly better functional recovery at 2 weeks, but not at 12 or 26 weeks, compared to those with persistently high apathy symptoms.
Correlates of Improved Apathy
Because these findings supported our hypothesis that improved apathy symptoms were associated with better functional recovery than persistent symptoms, we then examined clinical and demographic correlates of improved apathy. Delirium scores were lower and MMSE scores higher at both baseline and week 2 for those with improved apathy than for those with persistently high apathy; no other demographic or clinical features (including neurological illness, benzodiazepine use, or opiate use) differed significantly between these two groups. Baseline delirium scores and MMSE for the “high-low” and “high-high” apathy groups were 4.0 (SD=2.0) versus 8.6 (SD=5.6) and 24.9 (SD=2.8) versus 22.1 (SD=3.7), respectively. Adding these terms to the model did not change the strength of the difference between improved and persistent apathy symptoms in terms of functional outcomes (data not shown).
We also examined whether AES scores in the overall sample changed in concert with cognitive or depressive symptom scores between baseline and week 2. However, we found small and nonsignificant correlations of change in AES score with change in MMSE score (r=0.14, n=109), change in Delirium Rating Scale score (r=0.13, n=111), and change in HAM-D score (r=−0.08, n=112). Thus, changes in apathy symptoms between baseline and week 2 were independent of changes in cognition, delirium, or depressive symptoms.
DISCUSSION
In this study, we found a high rate (37%) of clinically significant symptoms of apathy in elderly persons after a hip fracture, comparable to rates in samples of elderly patients with neurological disease and considerably higher than a prevalence of 1%–2% in community elderly.
7 Supporting our first hypothesis, baseline apathy scores predicted functional recovery in the 26 weeks after hip fracture; poorer participation in physical and occupational therapy in those with high apathy scores may account in part for the poorer functional outcome. Additionally, older age and more cognitive impairment at baseline predicted poorer functional recovery. Apathy symptoms correlated moderately with delirium, depression, and cognitive measures, commensurate with prior research.
15 We also found that, among those with initially high apathy symptoms, by 2 weeks later one-third had improved.
Supporting our second hypothesis, improved apathy symptoms were associated with a better functional recovery. Patients with lower levels of delirium symptoms and better cognitive function were more likely to demonstrate improvement in apathy over the first 2 weeks. The difference between improved and persistent apathy groups in terms of functional outcome was no longer apparent by 12 or 26 weeks postfracture; this may reflect that improvements in apathy in the acute postfracture period have only an acute effect on function (i.e., during rehabilitative efforts). For example, later events, such as a medical incident or psychosocial problems, could have obscured the relationship between these acute improvements in apathy and long-term functional outcome.
These findings are important for clinicians treating elderly patients who have suffered disabling medical events. In this setting, apathy symptoms are common and potentially diminish over a relatively short time. This point is important because if apathy can improve, leading to better functional recovery, then apathy may be an important potential target for detection and intervention in the medical setting.
29 Published interventions for apathy have included stimulants and cholinesterase inhibitors, though these agents appear to have modest efficacy.
9 Another potential treatment in the context examined here (acute disabling medical events) is medical rehabilitation, providing an antiapathy effect similar to that posited for the apparent antidepressant effect of rehabilitation in elderly persons.
30 –
31 The high-intensity activity in this intervention may have an inherent antiapathy effect,
32 and successful rehabilitation alleviates functional disability and thus improves sense of control.
33One important limitation was our lack of brain imaging in this sample. In our study, the association of apathy with measures of attention and executive function but not memory suggests a prefrontal cortical association, as suggested by some
34 but not all
35 studies of apathy and neuropsychological function in the context of depression. Neuroimaging will be a critical part of future research delineating the neurobiological underpinnings of apathy following disabling medical events.
Another important limitation of the sample is its naturalistic nature, which prevents us from causally connecting apathy with subsequent functional outcome or understanding why some initially apathetic individuals improved (possibilities include the effect of rehabilitative efforts or the passage of time—regression to the mean). Thus, future research on apathy should further examine its persistence over time and should include clinical trials to treat apathy. It would be appropriate for clinical trials of pharmacologic and nonpharmacologic treatments in disabled medically ill elderly (not only those with neurological conditions) to systematically test the effects of various interventions on apathy and resultant benefits for functional recovery and other quality of life outcomes. A final limitation of the current study is that we did not have a prefracture assessment of apathy, which would be desirable (though difficult in terms of feasibility) for studies of apathy arising in the context of medical events.
In summary, we found a high rate of apathy symptoms in this non-neurological sample of acutely disabled medically ill elderly. Apathy symptoms appeared to have a negative impact on functional recovery. Improvement in apathy symptoms in a significant minority of patients, with concomitant improved functional recovery, generates the hypothesis that apathy symptoms could be a target of treatment with the goal of improving functional outcomes after disabling medical events.
Acknowledgments
The investigators would like to acknowledge the staff of the UPMC Shadyside Hospital and UPMC Presbyterian Hospital for their efforts with this study. This research was supported by NIMH grants K23 MH64196, K23 MH67710, P30 MH52247, P30 MH71944, and MH 072947; the UPMC Endowment in Geriatric Psychiatry; and the John A. Hartford Center of Excellence in Geriatric Psychiatry at the University of Pittsburgh. Dr. Lenze has received grant/research support from Novartis, Johnson & Johnson, and Forest. Dr. Munin has received grant/research support from Allergan, Inc. Dr. Whyte has received research support from Pfizer. Dr. Reynolds has received grant/research support from GlaxoSmithKline, Forest, Pfizer, and Lilly.