T raumatic brain injury (TBI) is a growing national health issue characterized by comorbid neuropsychiatric disturbances of mood, behavior, and cognition for which there are many proposed treatments but varying degrees of evidence. Approximately 1.4 million Americans sustain a TBI each year, which is likely an underestimate of the true incident burden.
1,
2 Considering a peak injury age of 15 to 24 years, there is a substantial load of chronic disability.
2 –
5 As such, TBI results in significant morbidity, mortality, and a massive economic toll, with U.S. costs estimated to be 60 billion dollars in 2000.
1,
2,
6 Traumatic brain injury is defined as a blow to the head or penetrating head injury that disrupts normal brain function.
6,
7 It is classified according to whether or not the skull has been breached (penetrating versus nonpenetrating) and the severity of the initial impairment (mild, moderate or severe).
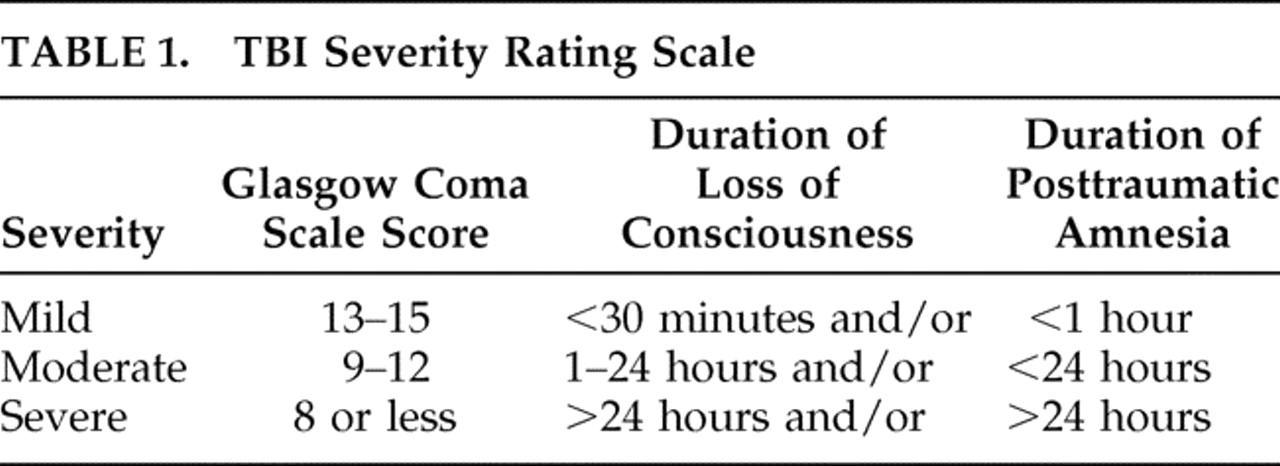
3,
8 –
10 Severity is typically determined by the presenting Glasgow Coma Scale (GCS) and the duration of loss of consciousness and posttraumatic amnesia (
Table 1 ).
1,
8,
10,
11 Although mild TBI is most commonly diagnosed, there is active debate regarding whether
loss of consciousness or an
alteration of consciousness is the necessary criteria, which likely has diagnostic, treatment, and prognostic implications.
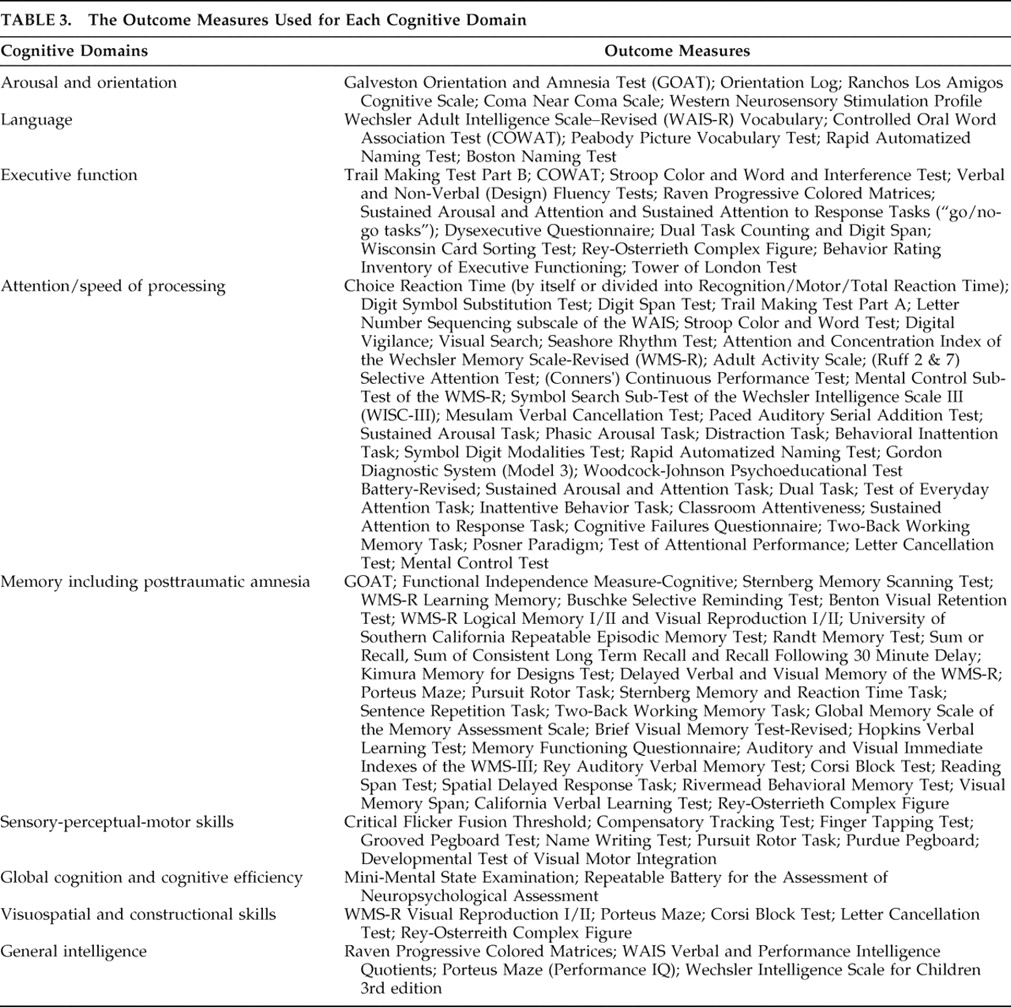
Cognition is divided into well-recognized but often overlapping domains of attention, executive function, memory, language, visuospatial and constructional abilities, and sensory-perceptual-motor skills.
4 Cognitive dysfunction can occur in multiple domains following TBI. These disturbances result from the combined effects of diffuse and focal damage typically to the anatomically vulnerable frontotemporal areas; thus memory, attention, and executive function deficits are most commonly observed.
4,
11 –
13 Cognitive impairment is the most common chronic sequelae of TBI which can result in more persistent disability than the physical injury.
13 Sources have reported that TBI severity correlates with cognitive and functional outcomes.
3,
10,
12,
14 For example, one prospective study (N=87) demonstrated that TBI severity moderately correlated with neuropsychological functioning (standardized coefficient=0.36, p<0.05) which correlated with functional outcome (standardized coefficient=0.52, p<0.05).
15 However, a key finding in this study was that cognitive impairment, particularly information processing speed, mediated the relationship between injury severity and functional decline (standardized coefficient=0.68, p<0.05).
16 This suggests that the restoration of functional autonomy is highly dependent on the resolution of cognitive deficits. However, there are no medications with an approved indication for treating TBI related cognitive impairment.
The purpose of this article was to systematically review the proposed psychopharmacological treatments for post-TBI cognitive impairment. We focused on commonly prescribed psychotropic medications to determine the relationship between pharmacological intervention and cognitive improvement in TBI survivors.
METHODS
Relevant articles published between 1950 and January 2008 were identified using Ovid MEDLINE. The following search terms were used: traumatic brain injury (then adding brain injuries to the populated list); antidepressive agents ; selective serotonin reuptake inhibitors (then adding serotonin uptake inhibitors to the populated list); antipsychotics (then adding antipsychotic agents to the populated list); CNS stimulants ; benzodiazepines ; mood stabilizers (then adding valproic acid, antimanic agents, carbamazepine, and lithium to the populated list); and cholinesterase inhibitors . A second search from 1950 to October 2008 was later performed using dopamine agonists (then adding bromocriptine to the populated list); amantadine ; and L - dopa . All terms were combined with traumatic brain injury/brain injuries .
We excluded nonhuman studies, non-English articles, reanalyses of previously published data sets, and case reports with fewer than five TBI subjects. We also excluded studies using the Glasgow Coma Scale as the only “cognitive” measure because it is not classically used as a cognitive outcome measure in the neuropsychological literature. Review articles detected by our literature searches identified additional sources.
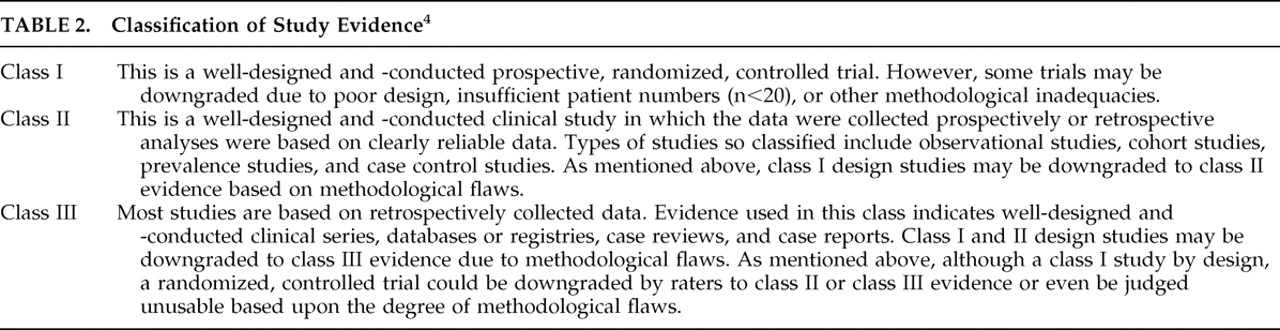
Each study was reviewed by both authors and independently classified according to the grading system utilized by Warden et al.’s
4 Neurobehavioral Guidelines working group, which adapted their system from the Brain Trauma Foundation’s Guidelines for the Management of Severe Head Injury. Each study was graded from class I to class III, with class I status being reserved for well-designed, double blind, placebo-controlled trials with reasonable subject recruitment (
Table 2 ). A consensus conference was held to resolve differences in the classification schema for select articles.
Cognitive domains were defined and grouped according to the divisions noted by Warden’s 2006 review which included attention, executive function, memory, language, sensory-perceptual-motor skills, and visuospatial and constructional abilities.
4 In addition, we included measures of global cognition, general intelligence, and arousal/orientation since they are relevant to TBI cognitive outcome. Funding sources were noted, and studies were considered “unfunded” if no mention of funding was made in the article. The length of the study was determined by the amount of time subjects were exposed to the pharmacological intervention.
DISCUSSION
Given the sobering morbidity and economic sequelae of TBI, the dearth of well-designed randomized, placebo-controlled trials guiding the use of medications for TBI-related cognitive dysfunction is disheartening. Only 32 studies met criteria for review, of which 27 were prospective. Within this group only four achieved class I criteria, and with the exception of one valproate study, all enrolled fewer than 40 subjects.
While there is clearly a need for more well-designed randomized, placebo-controlled trials, current literature has the most evidence supporting the treatment of attentional impairments with stimulants, memory impairments with stimulants and cholinesterase inhibitors, and executive impairments with stimulant and nonstimulant dopamine enhancing agents. Nine of 10 studies observed multidimensional cognitive improvement with methylphenidate and six of seven studies observed similar improvements with dopamine enhancing agents. Stimulants have also been associated with an approximate 25% decreased length of hospitalization in moderate to severe TBI survivors.
20,
25 Moreover, treatment of cognitive impairment with stimulant or nonstimulant dopamine enhancers is associated with improved functional performance.
20,
25,
30,
32 Although the data are not nearly as robust, four of five studies using acetylcholinesterase inhibitors also report cognitive improvement.
The results are highly mixed for all other psychopharmacological interventions. Antidepressant agents may have a role in treating depression-related cognitive dysfunction. However, these studies are limited by very small sample sizes using multiple outcome measures, the usual absence of a placebo group, and a failure to use multivariable modeling to determine the effect of these medications on cognition independent of mood resolution. While valproate and benzodiazepines may be cognitively neutral agents in TBI patients, the current literature does not support their use or the use of other mood stabilizers or antipsychotics for post-TBI cognitive deficits. Moreover, the only randomized, placebo-controlled trial of valproate did not even demonstrate prophylactic seizure benefit and actually showed a trend toward increased mortality.
47There are several limitations of this review. Our selection methods may have been too conservative given that only 32 of 561 abstracts were analyzed. Furthermore, our review, like all reviews, is inherently limited by the search terms we utilized. Had we expanded our search, we would have noted many other novel treatments such as
N -methyl-
D -aspartic acid (NMDA)-receptor antagonists, statins, progesterone, erythropoietin, minocycline, kinin antagonists, toll-like receptor antagonists, synthetic cannabinoids, magnesium, cyclosporine-A, and thyrotropin releasing hormone which have all been studied for their possible attenuating effects of subsequent neurochemical, cellular, and metabolic alterations following the primary TBI insult.
52 However, the purpose of this article was to focus on the cognitive effects of commonly prescribed psychotropic medications used “off-label” for TBI. We believe the studies we selected represent the most relevant published data for prescribing clinicians.
Treating TBI cognitive sequelae is complex and can be conceptualized along a continuum from acute to chronic care. TBI patients often have a variety of neuropsychiatric impairments and other comorbidities such as seizures, which are associated with cognitive impairment. As such, the acute and chronic management of TBI is interdisciplinary. This patient population often requires pharmacotherapy and/or rehabilitation services, which may also have cognitive benefits not addressed by this review.
Despite the propensity of certain focal areas to be injured with somewhat predicable neuropsychological disturbances, TBI is highly individualized. Subsequent neuropsychological impairments are dependent on biochemical disturbances, lesion locations, pre- and post-TBI neuropsychological status, and injury severity. The heterogeneity of brain injury certainly impacts therapeutic response to any given agent. This highlights the need for more controlled trials with narrower samples, which unfortunately limits generalization; however, this approach may lead to better treatment algorithms for specific TBI phenotypes.
Another limitation that future studies need to address is the multitude of neuropsychological testing utilized in these studies. Given that the long-range clinical goal is to restore functional status, perhaps the best approach is for investigators to choose neuropsychological measures that are consistently associated with functional outcomes in multivariable models. A recent review by the Committee on Research for the American Neuropsychiatric Association identified the Mattis Dementia Rating Scale-memory component and the Hopkins Verbal Learning Test as being particularly sensitive to memory-related functional impairment and the Executive Interview, Mattis Dementia Rating Scale-conceptualizations component, and Shipley Institute of Living Scale as being particularly sensitive to executive-function related functional impairment.
53 If the goal is to restore or preserve autonomy, then it only makes sense to use the measures that best correlate with functional status independent of mood, vigilance, and demographic confounders.
While our review suggests that dopaminergic psychotropic medications impart the most cognitive benefit to TBI patients among the commonly prescribed medications, there is a mounting volume of information regarding “second injury” processes involving the role of neuronal inflammation and apoptosis as molecular targets for future pharmacological intervention. Promising areas of study are currently measuring the effect of novel pharmacological agents in reducing inflammation while providing neuroprotection and brain remodeling via angiogenesis, neurogenesis, and synaptogenesis. We refer interested readers to a recent review of available literature by Vink and Nimmo
52 as well as to www.clinicaltrials.gov (search term
traumatic brain injury ) where they can find planned and in-progress Phase 1, 2, and 3 randomized clinical trials of NMDA-receptor antagonists, synthetic cannabinoids, progesterone, erythropoietin, and human growth hormone among other novel medications. This appears to be an exciting area of investigation that may yield benefits beyond classic psychopharmacological approaches.