A ggression is one of the most common consequences of traumatic brain injury (TBI). Prevalence estimates of post-TBI aggression range from 11%
1 to 34%,
2 likely due to differing samples and definitions. However, the phenomenology of post-TBI aggression is not yet well defined.
3 Aggression may manifest as verbal and/or physical aggression, but it is unclear if differing expressions of aggression are distinct syndromes or if they constitute a continuum of symptoms. Understanding the phenomenology and expressions of aggression in post-TBI aggression may help investigators with treatment and prevention. Post-TBI aggression may be a symptom of delirium, mood disorder,
4 or personality change secondary to TBI.
3 Aggression interferes with rehabilitation and is a major cause of burden to both the patient and caregivers.
5 Understanding the correlates and predictors of aggression may provide important clues for the prevention and treatment of post-TBI aggression, thus improving the rehabilitation potential in the crucial early post-TBI period.
Prior studies have shown that post-TBI aggression is correlated with depression, frontal lobe lesions, poor pre-TBI psychosocial functioning, and a history of alcohol and substance abuse.
2,
6 –
8 However, most of these studies focused on subjects who were several months to years postinjury. There is only one published study of aggression in the first 3 months after injury, which reported that severe TBI patients had cognitive and behavioral problems, but not emotional problems, when compared with mild to moderate TBI patients.
7 In the current study, we present preliminary cross-sectional findings on the prevalence and that correlates of aggression within 3 months of TBI in participants who are not delirious. We hypothesized that aggression would be common after TBI, aggression would include both verbal aggression and physical aggression, and correlates of aggression would include major depression, substance abuse, and adult/childhood behavior problems.
2,
3We sought to examine aggression in the first 3 months of TBI and characterize its severity and association with psychiatric diagnoses in adults with a first-time closed-head injury. This study is part of a larger ongoing study to determine prevalence and risk factors associated with the development of psychiatric disorders after TBI. The results presented here are thus preliminary.
METHODS
We performed an observational prospective study of the prevalence of aggression in the 3 months following TBI in a cohort of participants recruited within 3 months of trauma. We assessed the prevalence and subgrouping of aggression symptoms. We then examined the correlates of post-TBI aggression in a nested case-control design.
Participants and Procedures
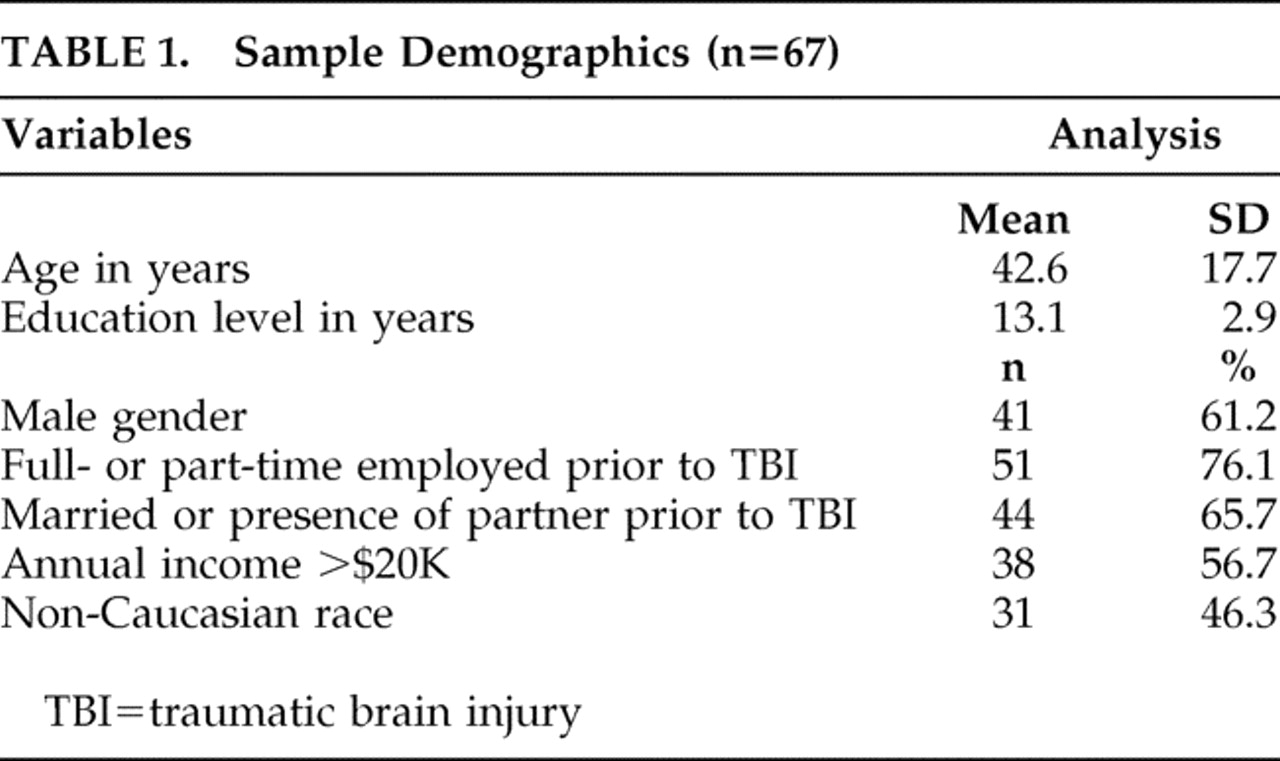
A total of 107 patients with first-time closed-head injuries were recruited within 3 months of trauma from the acute trauma unit of the Johns Hopkins Hospital and the Brain Injury (rehabilitation) Unit of Kernan Hospital at the University of Maryland. Evaluations were completed only on participants who were able to give informed consent; we evaluated the ability of participants to give informed consent based on their treating physicians’ opinions and based on the abilities of the participants to accurately summarize the study and their roles in it. All participants received two study evaluations within 3 months of the TBI. The first evaluation (V0) assessed lifetime history of psychiatric problems and pre-TBI psychosocial functioning in those participants who were able to provide written informed consent within the first 2 weeks of trauma. The second evaluation (V1) of these participants was done approximately 3 months postinjury to assess psychiatric problems and psychosocial functioning after traumatic brain injury. However, for participants who were unable to give consent within the first 2 weeks, both pre-TBI and post-TBI status were assessed at the time they were able to provide informed consent, but within 3 months after the TBI (i.e., for these participants, V0 and V1 data were collected at a single visit corresponding to the V1 visit). Information from a collateral informant was collected whenever possible on both pre-TBI and post-TBI status on all psychosocial measures.
Of the 107, 40 participants did not return for the follow-up visit and were therefore excluded from this analysis. Of the 40 excluded, 25 could not be contacted because of incorrect address and telephone numbers, eight refused to participate, five had unstable medical problems, one was incarcerated, and one had anoxic brain injury.
For the purposes of the study, TBI was defined as having at least one of the following : (a) clear history of loss of consciousness; (b) Glasgow Coma Scale score less than 15; and/or (c) evidence of trauma (contusion or hemorrhage) on computerized tomography (CT) scans done as part of clinical workup. Other inclusion criteria included the ability to provide consent personally, age at least 18 years old, and admission to the hospital for evaluation of head trauma. Exclusion criteria included prior TBI, an open-head injury (e.g., a displaced skull fracture or a gunshot wound), or a history of any other type of brain illness (e.g., stroke, seizure, or encephalitis). The study was approved by the institutional review board of both universities.
Measures
All measures were administered by a neuropsychiatrist (VR) except the cognitive tests, which were administered by the study research coordinators (JS and MB).
Aggression
The Overt Aggression Scale
9 was used to assess verbal and physical aggressive behavior. This scale has two sections. The first assesses four types of aggressive behavior: verbal aggression, physical aggression against objects, physical aggression against self, and physical aggression against others. The severity of each subtype can be rated using a weighted score: verbal aggression (1–4 points), physical aggression toward objects (2–5 points), physical aggression toward self (3–6 points), and physical aggression toward others (3–6 points), with a range of 0–21 for the total Overt Aggression Scale score (higher scores indicate more severe aggression). The second section rates interventions provided by staff at the time of the incident. As the focus of the study is to assess prevalence and correlates of aggression after TBI, only the first section of the scale was administered at V1. The Overt Aggression Scale has been validated in adult and pediatric inpatients with neuropsychiatric illness and violent behavior.
10 –
12 Other researchers have also noted significant correlation between the Overt Aggression Scale and the Aberrant Behavior Checklist Community Scale irritability subscale in a study of outpatient youths with aggression.
13Psychiatric Diagnoses
Axis I psychiatric diagnosis was determined using the Structured Clinical Interview for DSM-IV axis I disorders (SCID-I)–Clinician Version.
14 Diagnoses such as impulse control disorder and intermittent explosive disorder, which are probably relevant to TBI aggression, were not obtained because the SCID-I does not contain these diagnoses.
Severity of TBI
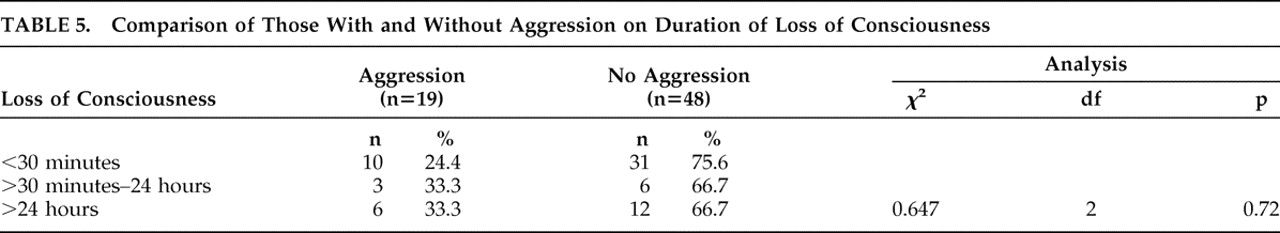
The severity of TBI was determined by the Glasgow Coma Scale (GCS), the most widely used instrument for quantifying TBI severity. The GCS is administered by the trauma staff or the emergency room personnel in their initial evaluation and has a range of 3–15. GCS scores of 3–8 are considered severe TBI, 9–12 moderate TBI, and 13–15 mild TBI.
15 All those determined to have mild TBI, as defined by GCS, also met the mild TBI criteria of the American Congress of Rehabilitation.
16 The American Congress of Rehabilitation Medicine (ACRM) defines mild TBI as traumatically induced physiological disruption of brain manifested by
at least one of the following : (a) any period of loss of consciousness; (b) any loss of memory for events immediately before or after the accident; (c) any alteration in mental state at the time of the accident (e.g., feeling dazed, disoriented, or confused); and (d) focal neurological deficit(s) that may or may not be transient. However, the duration of posttraumatic amnesia should not be greater than 24 hours, the duration of loss of consciousness should not exceed 30 minutes, and the initial Glasgow Coma Scale (GCS) score should range from 13–15 after 30 minutes postinjury.
Medical Comorbidity
Medical comorbidity was assessed using the General Medical Health Rating scale.
17 This rating, which ranges from 1 (poor health) to 4 (excellent health), provides a global assessment of a person’s medical problems and medications. Data on the number of medications used by each patient were collected, but not the type of medications.
Psychosocial Functioning
Participants’ pre- and post-TBI psychosocial functioning was assessed using the Social Functioning Exam and the Social Ties Checklist.
18 Both these scales have been used in prior TBI studies.
19,
20 Scores on the Social Functioning Exam and the Social Ties Checklist range from 0 (greatest satisfaction) to 1 (least satisfaction). The reliability and validity of these instruments have been demonstrated in patients with brain injury.
21Family History of Psychiatric Illness
Family history of psychiatric illness was assessed using the Family History Screen instrument.
22Behavior and Legal Problems
Pre- and post-TBI behavior and legal problems were also analyzed. Childhood behavioral problems were defined by the presence of two or more of the following: suspension from school, expulsion from school, setting fires, cruelty to animals, and/or destroying property. Adult behavioral problems were defined as being fired from work, fights, or violent behavior that interfered with interpersonal relationships and occupational or social life. Legal problems were defined as the presence of one or more of the following: arrests, incarcerations, jailed, or being placed on parole or probation. Both pre- and post-TBI legal status were included in the assessment.
Cognitive Tests
Neuropsychological tests were administered to all study participants at V1. The battery consisted of the Mini-Mental State Examination (MMSE)
23 ; the National Adult Reading Test
24 ; verbal fluency (letters “s” and “p”) and category (animals and supermarket)
25 ; Hopkins Verbal Learning Test—Revised
26 ; Brief Visuospatial Memory Test—Revised
27 ; Trail Making test
28 ; Stroop Color and Word Test
29 ; Brief Test of Attention
30 ; and the Wisconsin Card Sorting Test.
31Neuroimaging
All participants had a head CT scan performed as part of routine clinical care. The CT results were categorized as presence or absence of lesions in different brain regions (i.e., right, left, bilateral frontal, temporal, parietal, occipital, subcortical).
Data Analysis
Participants were categorized as having “aggression” if they endorsed any of the subtype anchor questions on the Overt Aggression Scale. Those who endorsed a screening question for a particular subtype were then asked follow-up questions to quantify the severity of aggression on that subtype.
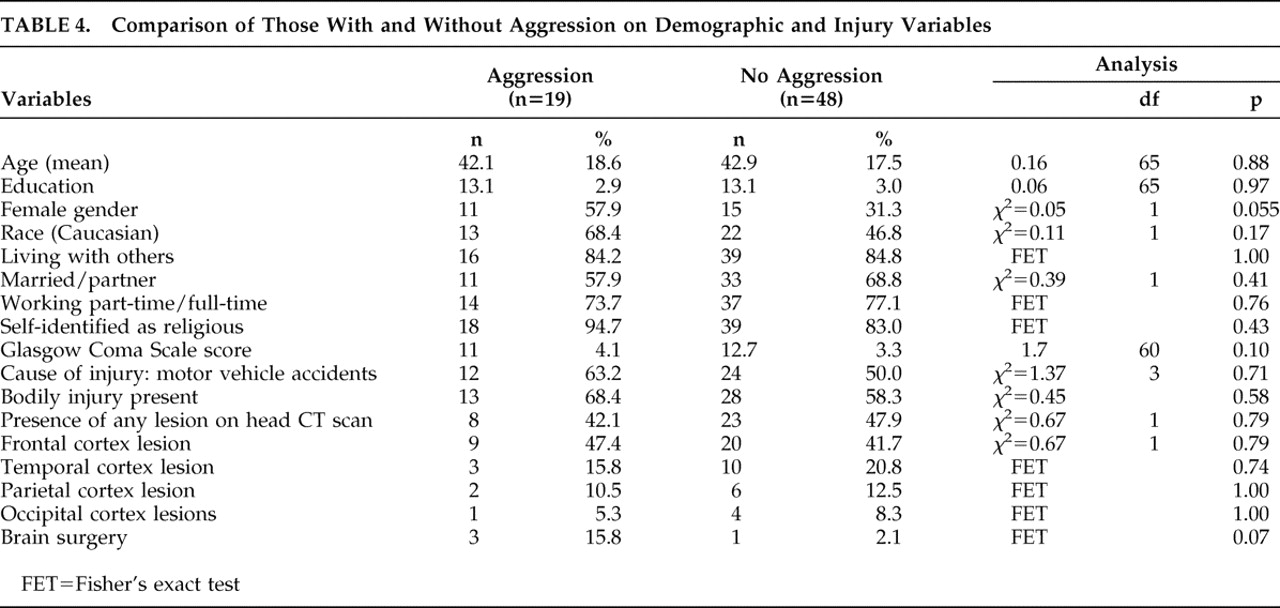
Descriptive statistics were calculated for all participants and for subgroups stratified by post-TBI aggression status. The significance of group differences (two-tailed) on individual variables was compared using Pearson’s chi-square and Fisher’s exact test for categorical variables and Student’s t test for continuous variables. The criterion for statistical significance was set at p<0.05.
To assess the strength of the relationship between aggression and the demographic and clinical factors, we conducted univariate logistic regression analysis with presence/absence of aggression as the dependent variable. On comparison of the two groups, those variables that were statistically significant and those that trended toward significance were included as independent variables in the univariate regression analyses. Significance levels were set at p<0.05.
DISCUSSION
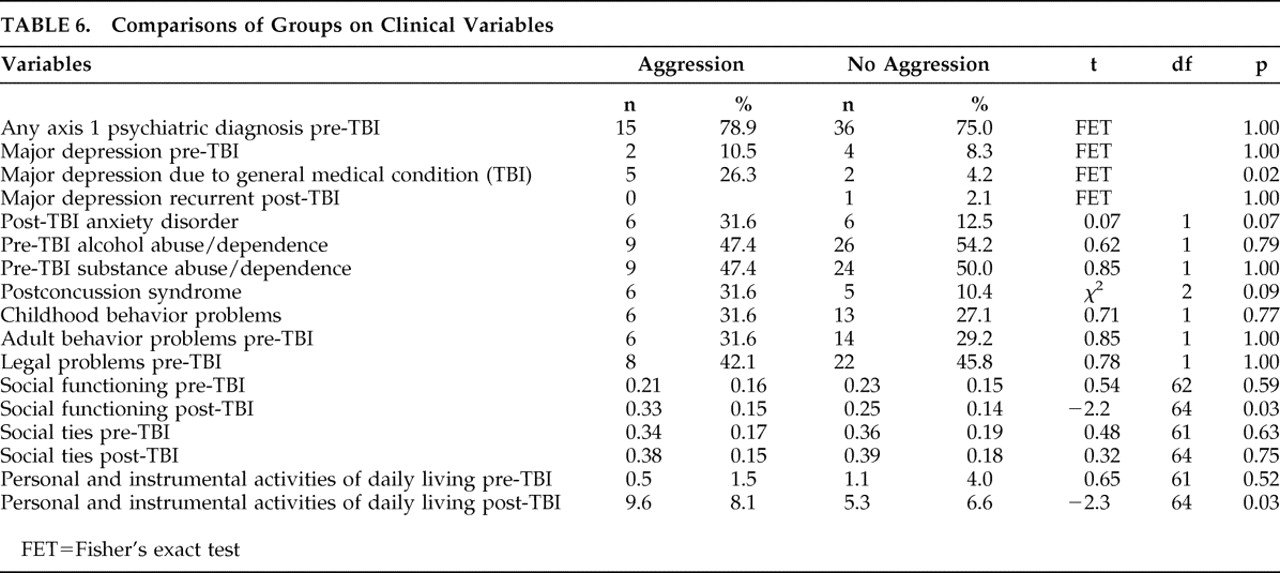
In a nested case-control study of TBI patients during the first 3 months following injury, we found that verbal aggression was quite prevalent, but physical aggression was virtually absent. Aggression in the post-TBI period was associated with impaired post-TBI psychosocial functioning, new-onset major depression post-TBI, and increased dependence in activities of daily living. Interestingly enough, recurrence of pre-TBI major depression was not a predictor of aggression.
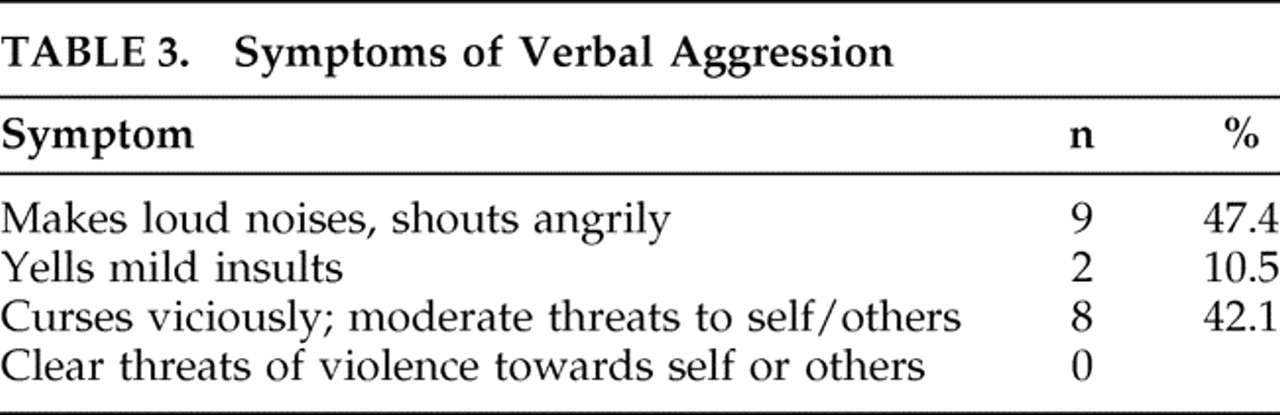
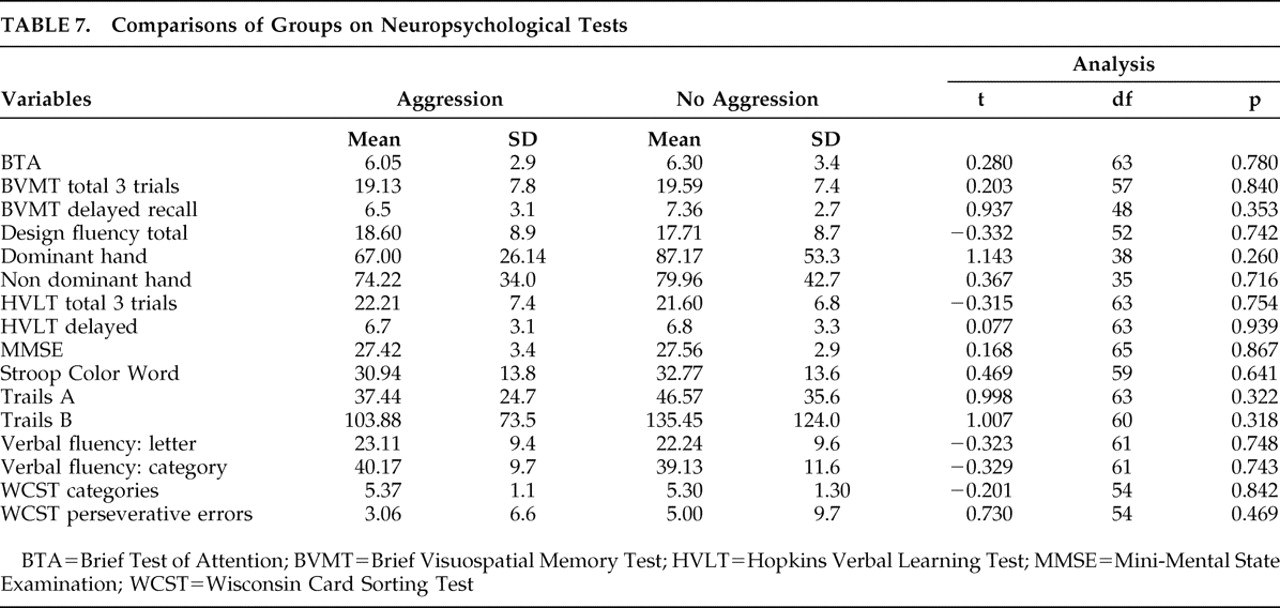
We report several significant negative findings. Only one of the 67 participants exhibited physical aggression, and this was in a milder form (aggression only toward objects). None exhibited physical aggression toward self or others. We observed no significant association between post-TBI aggression and several covariates reported in prior studies, including childhood behavior problems, adult behavior problems, legal charges, pre- or post-TBI substance abuse or dependence, neuropsychological tests or brain lesions.
The prevalence of aggression is similar to what is reported in the literature,
2,
8 as is the mix of TBI severity, with approximately 60% having mild TBI.
33 On reviewing the literature on the subtypes of aggression after brain injury, several studies have noted an association between brain injury and physical aggression.
34 –
37 Only one person reported a history of physical aggression in our study. Our findings are consistent with anecdotal reports on the predominance of verbal aggression after TBI.
38,
39 Dyer et al.
39 have also noted a higher prevalence of verbal aggression rather than physical aggression in a sample of TBI patients studied 6 months postinjury. This discrepancy in the literature could be due to differences in the definition of aggression, severity of TBI, and duration since TBI. Another possibility is that TBI aggression occurs in two forms: mild verbal aggression associated with mood disorder but not with preinjury or postinjury behavior problems, and severe physical aggression which can be more complex and associated with significant behavior and legal problems.
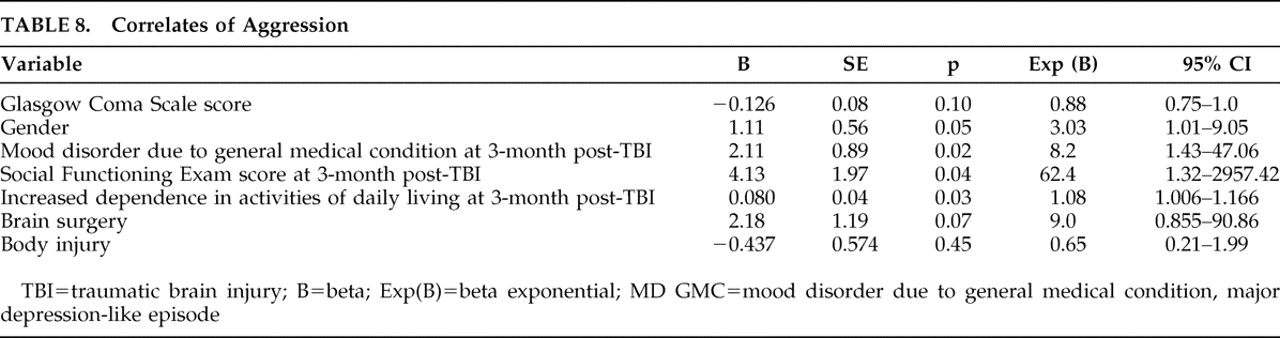
We did, however, observe that aggression was strongly associated with new-onset major depressive episodes which increased the risk of aggression by eightfold, but not with recurrent major depression. Possibly new-onset depression after TBI is a phenotypic variant of idiopathic major depression with verbal aggression as a presenting symptom. Several other studies
2,
8 have also noted a significant association between depression and aggression, but none of them have separated post-TBI depression as new-onset depression and recurrent depression. This is one of the strengths of our study.
Many researchers have also noted a relationship between aggression and premorbid characteristics such as childhood behavior problems,
6 adult behavior difficulties, legal problems
40 and substance abuse.
2,
41 However, we did not observe these associations in our cohort. All prior studies have examined global aggression, combining both verbal and physical aggression. The lack of an association in our study could be because our study observed only verbal aggression, which is likely to reflect a milder form of aggression than physical aggression. Thus, it is possible that mild (verbal) aggression is associated with post-TBI depression, while more severe (physical) aggression may be associated with adult and childhood behavioral problems and substance abuse. Our finding of the relationship between female gender and aggression (that trended toward statistical significance) was unexpected but not surprising, given our findings of a positive relationship between aggression and depressive syndrome. It is well known that females are at higher risk of developing major depression,
42 though this has not been established for TBI cohorts.
Our study also found a significant association between verbal aggression and post-TBI social impairment. Baguley et al.
8 have also noted a significant association between poor satisfaction with life and aggression 6 and 24 months post-TBI. This is not surprising as one might predict that aggression would be interfering with relationships and reintegration into the community. However, the increased risk of aggression in persons with poor social functioning is quite remarkable (62-fold increased), even higher than that of depression. This finding has significant therapeutic implications for the immediate post-TBI period, suggesting the particular importance of improving psychosocial support, strengthening social connections, and providing adequate resources via individual, group, and family therapy in reducing aggression. Studies have shown that psychosocial support and positive interpersonal relationships play an important role in reducing aggression.
43 An alternative explanation for our results is that early diagnosis and appropriate treatment of aggression might lead to better social and interpersonal functioning.
The significant association between aggression and increased dependence in activities of daily living has been reported before.
44 One possibility is that persons with TBI are resistant to assistance provided by their caregiver, perceiving assistance as an invasion of personal space. It is also possible that aggression may be associated with lack of motivation in the context of post-TBI depression with concomitant lack of interest in self-care and help from care providers. Alternatively, aggression may only be a confounder, as our results showed that those with bodily injuries were more likely to have increased dependency in activities of daily living.
The literature suggests potential brain mechanisms for post-TBI aggression. Tateno et al.
2 found frontal lesions to be associated with aggression and suggest that frontal lobe injury can cause damage to the ascending serotonergic pathways, which can contribute to the pathophysiology of both depression and violent behavior. As systematic analyses of neuroimaging data were not part of our study, we are unable to comment on this possibility. However, we found no significant difference between those with and without aggression on CT head scans, performed as part of clinical workup. Similarly, we also did not find any group differences on neuropsychological tests, and more specifically, on tests particularly sensitive to the frontal functioning system. Starkstein and Robinson
45 have pointed out that TBI aggression is probably secondary to loss of balance between inhibitory pathways in the prefrontal cortex and excitatory limbic structures that mediate mood. These mechanisms could explain our finding of an association between aggression and new-onset depression in the immediate post-TBI period. More sensitive neuroimaging tools such as functional MRI or diffusion tensor imaging scans may shed light on the brain mechanisms underlying TBI aggression.
Our findings do not prove that the aggression in the post-TBI period observed in our subjects was caused by the TBI nor that any association was due to the biological effects of brain injury. For example, bodily injury was associated with increased dependence in activities of daily living which was further associated with aggression. It is possible that bodily injury could be an important mediator of aggression in the population, but the small sample size did not allow for systematic assessment of mediators and interactions. Similarly, the lack of association between TBI severity and aggression suggests the possibility that factors other than TBI are responsible for aggression; conversely, it could also suggest that TBI itself (rather than TBI severity) is an important risk factor for aggression. The lack of control groups with exposure to non-TBI bodily injuries and/or no injury is a limitation in addressing these issues. Other limitations of the study include the assessment of a narrowly defined cohort of TBI patients. Our study sample included only those with first-time closed-head injury, clear history of loss of consciousness, and those who were hospitalized. These strict inclusion/exclusion criteria may limit generalizability. Further, medication data were not available, precluding any study of the association between medications and aggression. Antipsychotics, benzodiazepines, antidepressants, and opioid analgesics are often used in the acute TBI period and might be effective treatments for severe agitation, which could account for the absence of physical aggression in our cohort. Systematic analyses of brain imaging data were also not performed, and thus we were unable to assess the association of exact lesion location and aggression. Finally, aggression was assessed only once in the acute TBI period by self-reports, which are often associated with underreporting and minimization of symptoms. However, whenever collateral informants were available, the subjects’ history was always corroborated.
To the best of our knowledge, this is the second published study examining aggression in nondelirious patients within the first 3 months of TBI. Our finding of a significant association between poor psychosocial functioning and verbal aggression underscores the point that psychosocial support is an important aspect of emotional recovery and therefore should be an integral part of rehabilitation. Our preliminary finding that new-onset major depression after TBI, but not recurrent major depression, is associated with post-TBI aggression is novel and may have substantial implications for the phenomenology and treatment of post-TBI aggression.