I n 2001 suicide was the third leading cause of death among Americans ages 15–24, and tenth overall.
1 In addition to over 30,000 deaths, attempted suicide was the cause of over 425,000 hospitalizations.
2 As such, suicide is a serious problem in the United States.
A prominent risk factor for suicide is a history of depression,
2,
3 and brain imaging studies are now uncovering its neurobiological substrates. In particular, studies by Brody et al.,
4 Drevets,
5 and Mayberg et al.
6,
7 have implicated overactive circuits projecting from the subgenual cingulate gyrus (Brodmann’s area 25) in major depressive disorder. In addition, imaging studies on patients who have attempted suicide have shown low serotonergic function in the prefrontal cortex.
8,
9Our study retrospectively compared two experimental groups of 12 patients to 12 age- and gender-matched healthy subjects using brain SPECT imaging. In the first group, each patient ultimately committed suicide; the second group was randomly chosen from among a large patient database and matched to the suicide group by age and gender. Our goal was to explore in vivo brain differences in this unique sample in an effort to better understand the brain function of those who completed the act of suicide. We hypothesized finding low prefrontal and medial prefrontal activity among the suicide group coupled with increased rCBF in the subgenual cingulate gyrus indicative of depression.
METHODS
Experimental and Comparison Groups
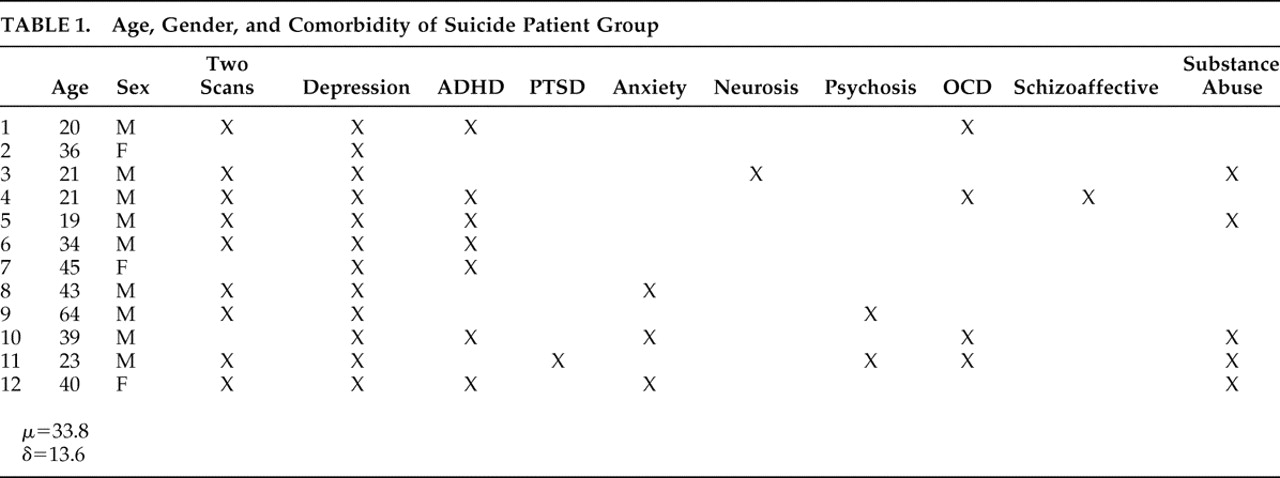
In an exhaustive search of a 21,000 patient database acquired over the years 1992–2007 at four neuropsychiatric clinics, we identified 12 right-handed patients (mean age=33.8, min=19, max=64) who had sought and subsequently underwent brain SPECT imaging for depression and who, subsequent to those scans, committed suicide. These 12 constitute the experimental group. Psychiatric diagnoses were based on DSM-IV criteria and the Beck Depression Inventory (BDI); all were confirmed by a licensed psychiatrist. In three cases patients in this group did not complete the BDI (mean=27.5, SD=11.97); however, these patients met DSM-IV criteria for depression and received a clinical diagnosis as such from a psychiatrist. Eight of the 12 suicide patients had been off medications for appropriate washout periods at the time of their scans. The four remaining patients are described as follows: one patient was taking a selective serotonin reuptake inhibitor (SSRI), an anticonvulsant, and an antipsychotic; one was taking an SSRI, an anticonvulsant, and a benzodiazepine; one was taking a serotonin-norepinephrine reuptake inhibitor (SNRI) and an antipsychotic; and one was taking an SNRI. The mean time from scan to suicide was 9.65 months (median=8 months, min=10 days; max=36 months). Nine of the 12 patients committed suicide within 1 year of being scanned. The relevant characteristics and comorbidity of all 12 patients who committed suicide are summarized in
Table 1 .
Our nonsuicide/depressed patient group was chosen randomly from the database described above, wherein scans were acquired over the same period. Using the characteristics of the patients who committed suicide, the nonsuicide patients were pooled into 12 corresponding groups based on age, gender, and comorbidity, and one subject was randomly chosen from each group. Thus the nonsuicide patient group had exactly the same comorbidity profile as the suicide group, shown in
Table 1 . All 12 nonsuicide patients completed the BDI (mean=29.6, SD=9.5). Nine of the 12 patients were scanned off medications after appropriate washout periods. The remaining three are described as follows: one patient was taking bupropion, a benzodiazepine, and an anticonvulsant; one was taking an SNRI and an antipsychotic; and one was taking an antipsychotic and an anticonvulsant.
No patients in the experimental group exhibited any neurological symptoms. All patients in this study had the procedure explained to them, at which time they gave informed written consent to have their scan and other information used in future anonymous research.
Our healthy brain comparison group likewise consisted of 12 right-handed age- and gender-matched adults. Over a 5-year interval, our comparison subjects were recruited in an institutional review board-approved study and were screened for head trauma, drug and alcohol abuse, and psychiatric (SCID), psychological (MMPI
10 ), and neurological (Shenkel’s Mental Skills Test
11 ) disorders among themselves and, via self-report, their first-degree relatives. In accordance with the aforementioned protocol, the procedure was explained to all healthy comparison subjects, who then gave informed written consent prior to their inclusion in the study.
SPECT Image Acquisition and Rendering
We used SPECT to capture functional images of regional cerebral blood flow (rCBF) as a surrogate for neuronal activity. For each scan, an age- and weight-appropriate dose of technetium-99m exametazime (Ceretec) was administered intravenously. We then acquired images of photon emission using a high resolution Picker (Phillips) Prism 3000 triple-headed gamma camera with fan beam collimators over a 15-minute interval. Data were acquired in 128×128 matrices, yielding 120 images per scan with each image separated by 3° (6.6 mm) spanning 360°, and Chang attenuation correction was performed using general linear methods. All scans were acquired using the same model camera with the same fixed parameters and were processed using the same methods.
Nine of 12 suicide subjects received two scans, one at rest and one during an attention challenge, while three received only a concentration scan with no resting scan; all nonsuicide patients and healthy comparison subjects received both resting and concentration scans. In all cases the two scans were acquired between 24 and 48 hours of each other. We acquired resting images in the following manner: subjects sat upright in a quiet, dimly lit room with eyes open, with the bolus injected at 10 minutes, whereupon they sat for an additional 10 minutes postinjection. We acquired concentration images during a 15-minute computerized go/no-go task measuring omissions, commissions, and reaction time: Connor’s Continuous Performance Test.
12 Subjects sat upright in the same light with the same acclimation time as in the baseline condition. We administered the injection 3 minutes into the Connor’s Continuous Performance Test. As the tracer is fully taken up by the brain by the second minute postinjection, we thus capture a “steady-state” image of the functional brain 4–5 minutes into the Connor’s Continuous Performance Test.
We processed all images using Odyssey software, orienting transaxial slices horizontal to the AC-PC line. Coronal, sagittal, and transaxial slice images (6.6 mm apart, unsmoothed) were then rendered in the Odyssey Step-20 software, which color-grades voxels based on their relative frequencies of photon emission. Scans were then converted to Analyze format and then coregistered and normalized to a standard anatomical space using SPM2 software. In order to minimize small aberrant but statistically significant results, we smoothed scans to 8 mm 3 .
Study Design and Data Analysis
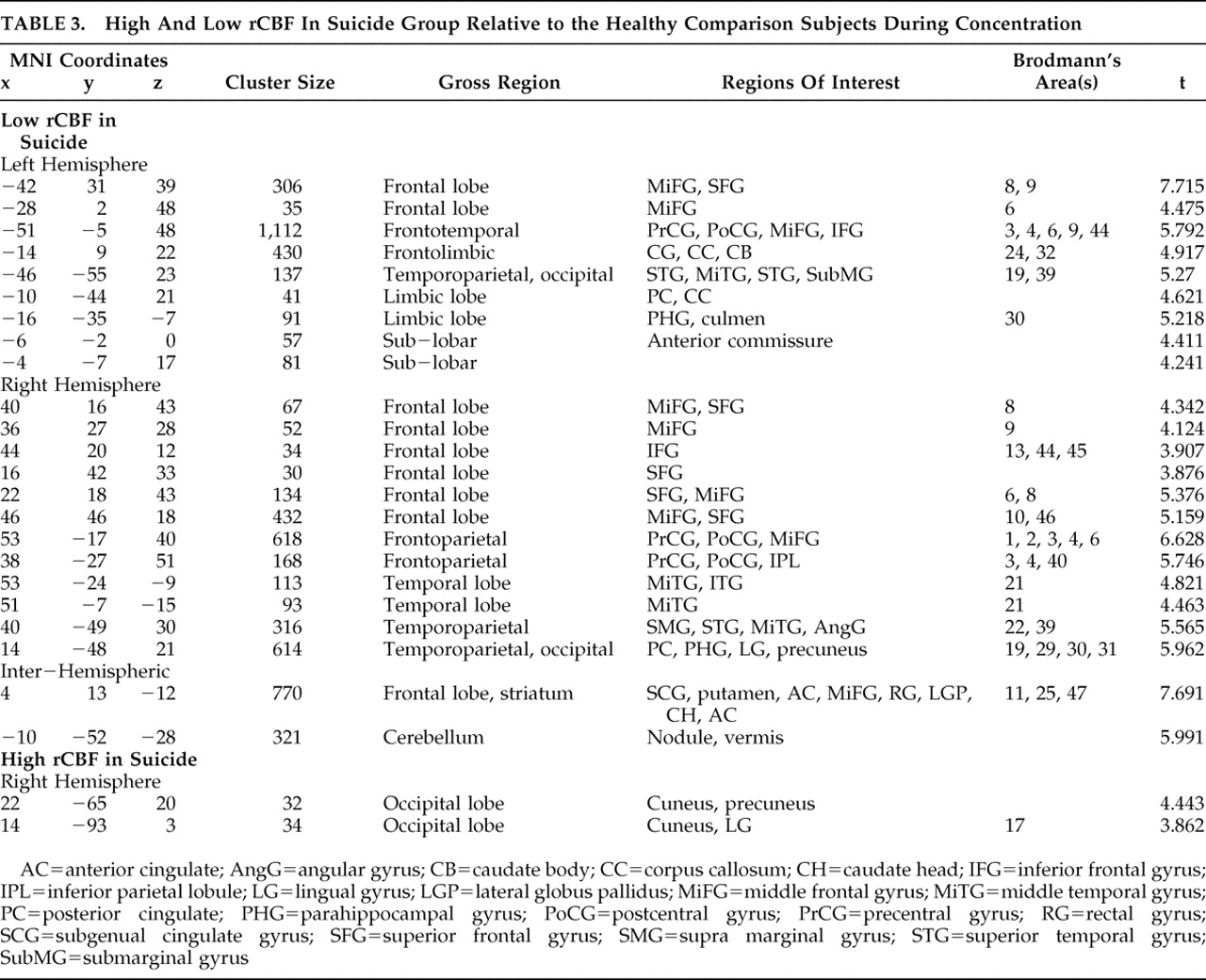
In comparing our suicide group to healthy brain comparison subjects, we adjusted all scan data for the effect of global image signal by using a Thresholded Mean Voxel Value in SPM2. We ran two different voxel-by-voxel one-tailed t tests in SPM2, one comparing nine suicide patients to healthy comparison subjects at rest (baseline), and one comparing 12 suicide patients during concentration. We then ran two additional analyses. First, we looked at only those nine patients who had received both resting and concentration scans, comparing them at concentration. Next, we looked at all 12 suicide subjects during concentration using dummy variable indicators to test whether the “concentration only” patients were significantly affecting the outcome. As our results remained robust, in addition to reporting the results from our analysis of nine resting scans, we additionally report findings from all 12 concentration scans. We then performed similar SPM2 analyses comparing nonsuicide depression patients with healthy comparison subjects and suicide with nonsuicide depression patients at both baseline and concentration, for a total of six comparisons.
In the suicide/healthy brain and nonsuicide depression/healthy brain subject comparisons, we used a significance threshold of p=0.001 (uncorrected for multiple comparisons), constraining output to retain only those significant differences comprised of a minimum of 30 contiguous voxels. We used a relative threshold mask of 80%, and voxels outside the brain were excluded from the analyses.
For the suicide/nonsuicide depression comparison, in order that we might better uncover any subtle differences between suicidal and nonsuicide depression, we relaxed the significance threshold to p<0.02. In these cases, we report uncorrected p values.
DISCUSSION
To our knowledge, this is the first in vivo study conducted on patients who have completed the act of suicide. Consistent with previous imaging studies on depressed subjects,
12 –
14 we found decreased rCBF in the prefrontal and parietal cortices in both the suicide and nonsuicide depression groups, also noting low activity in the anterior cingulate and orbital cortices indicative of the compromised executive functioning we would expect in severe depression.
A number of studies implicate the limbic circuitry and its dopaminergic pathways in depression.
15,
16 At the core of this circuit is the amygdala, which processes emotional stimuli and is involved in reward-based learning and reinforcement, in part through its projections to orbital (Brodmann’s area 11) and medial prefrontal cortical areas (Brodmann’s areas 12, 25, 47). Amygdala activation has an inhibitory effect on prefrontal areas, which in turn are modulated by dopaminergic inputs from the ventral tegmental area of the midbrain. In our study, both experimental groups showed normal bilateral perfusion in the amygdala but low perfusion in the ventral tegmental area, medial prefrontal cortex, and orbital cortex. This is particularly important given research indicating that amygdala activation unaccompanied by a release of dopamine to the medial prefrontal cortex may result in depressive symptoms such as blunted affect and anhedonia.
15In contrasting baseline and concentration results within each group, we found the perfusion deficits present at baseline are somewhat attenuated in the depressed group but exacerbated in the suicide group during concentration. For example, we noted that deficits in the middle and frontal gyri were better perfused in nonsuicide depression patients, but degraded in suicide subjects during concentration. We also found a large deactivation in the posterior cingulate of the suicide group. This may be an important difference between a typically depressed population and one with the potential for suicide, as path analyses by Stein et al.
17 identified an inverse relationship between activity in the posterior cingulate and amygdala. Down-regulation of the posterior cingulate would correspond to increased amygdala activity and thus greater medial prefrontal cortex inhibition, consistent with our findings.
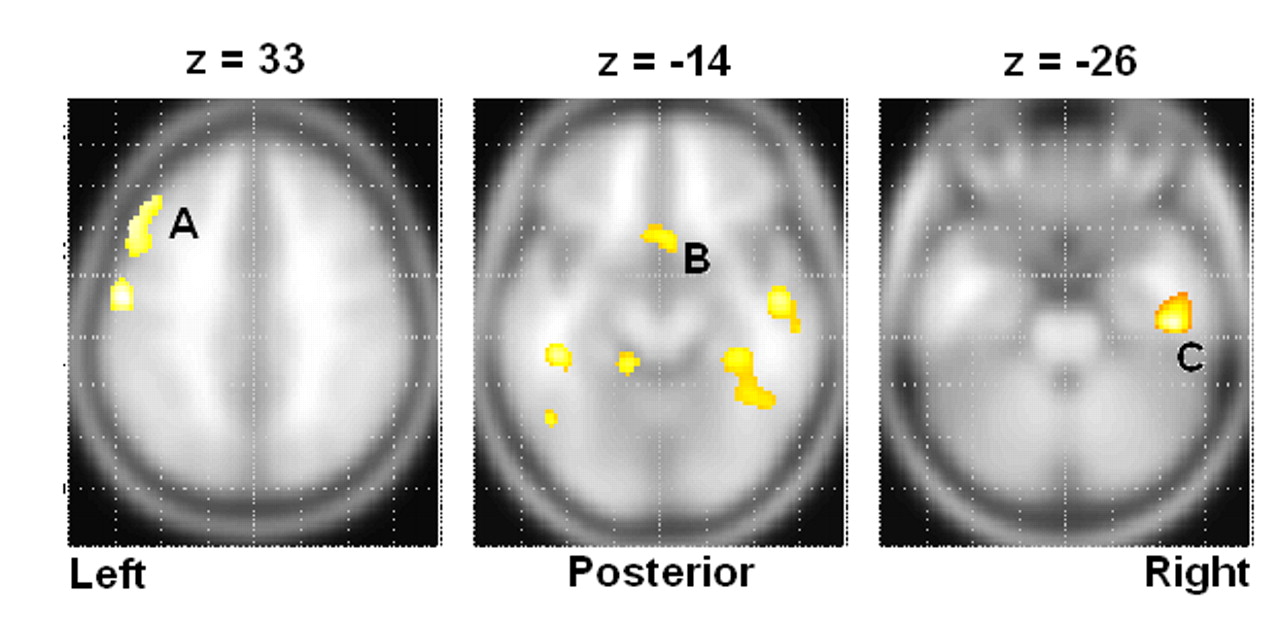
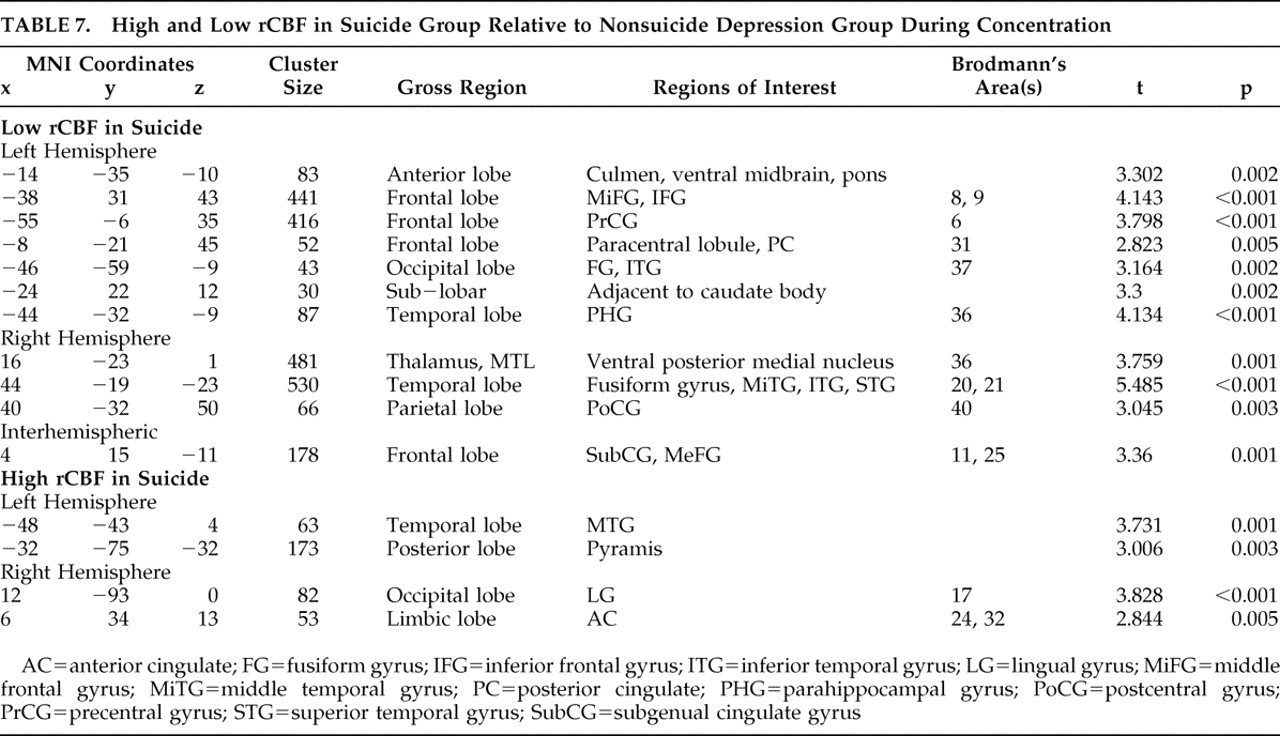
Other important differences between the suicide and nonsuicide depression groups emerged in our direct comparison of them (
Table 6 and
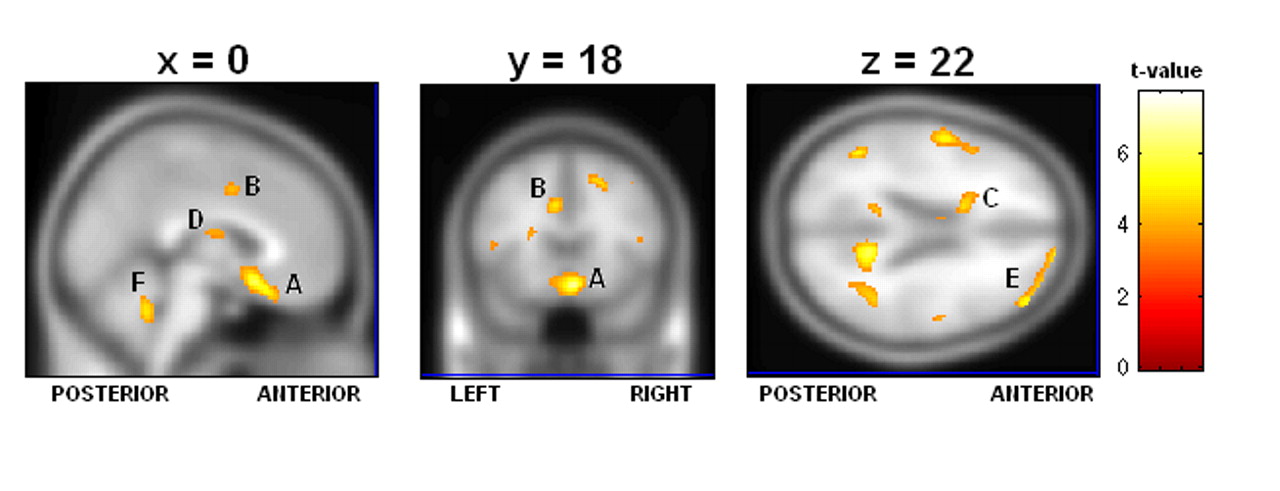
Table 7 ), particularly the low rCBF we saw throughout the frontal and limbic regions in suicide, which was greatly exacerbated in the concentration condition. We also found significant suicide hypoperfusion in the subgenual cingulate cortex (Brodmann’s area 25), a region inferior to the ventral cingulate and anterior to the paraterminal gyrus, the subgenual cingulate cortex. This area, coined the visceromotor control region by Neafsey et al.,
18 has projections to the brainstem and throughout the striatum, with reciprocal connections to the posterior cingulate, frontal and medial frontal cortices, hypothalamus, and amygdala.
19,
20 As such, Brodmann’s area 25 is thought to be critical in mood regulation.
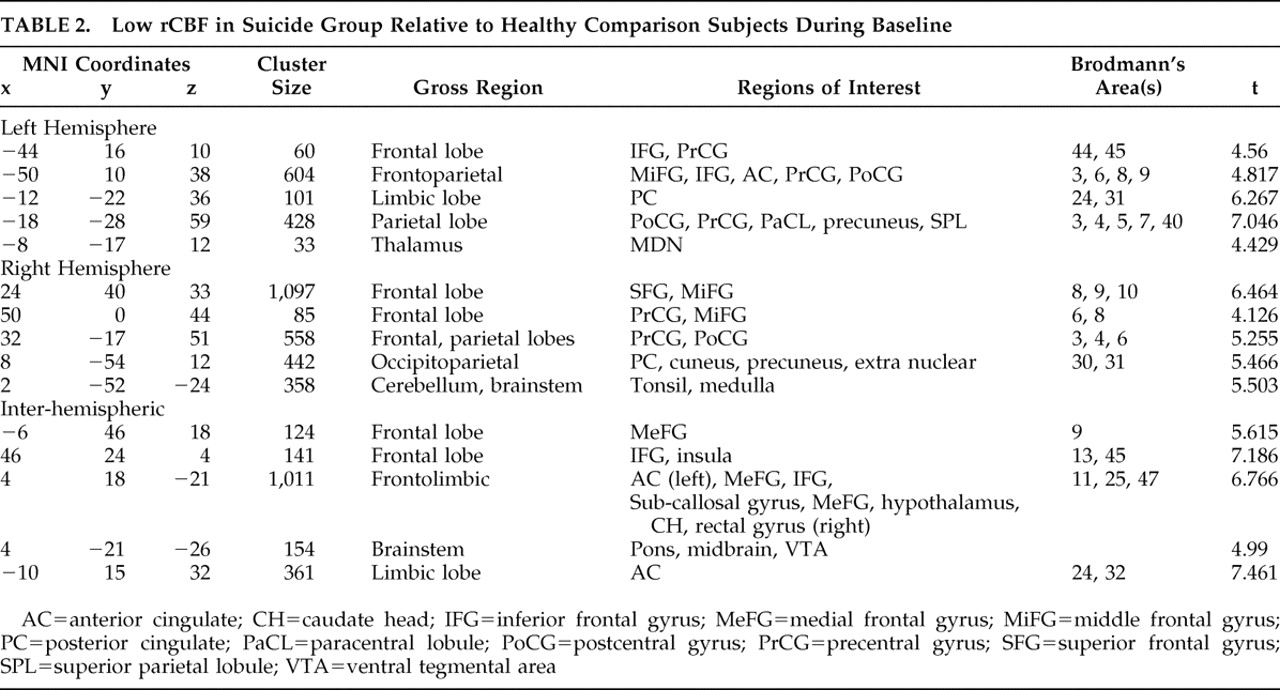
Brodmann’s area 25 hyperperfusion measured by PET has been associated with major depression.
4 –
7 However, our results are consistent with Skaf et al.
21, who found subgenual hypoperfusion in depressed patients with and without psychotic features using SPECT. PET studies by Kennedy et al.
22 and others have shown that a positive response to venlafaxine is associated with a reduction in Brodmann’s area 25 glucose metabolism. As we noted Brodmann’s area 25 hypoperfusion deficits only in our suicide group, our results indicate that severe Brodmann’s area 25 hypoperfusion may correspond with a class of treatment-refractory depression.
Two instances of high rCBF in the suicide group relative to the nonsuicide depression patients are additionally of interest. First, we note increased relative perfusion in the dorsal anterior cingulate cortex, Brodmann’s area 32. This area is part of the “limbic loop” and has excitatory projections to the posterior cingulate,
23 and activity here coupled with low posterior cingulate rCBF is indicative of dysregulation along this pathway. Second, we note increased right insular rCBF in the baseline condition. Phillips and Drevets
24 linked this area to ventral limbic pathways, and activations here may be related to a disruption in mood regulation.
There are a number of limitations to this study, primarily associated with the unusual heterogeneity of our study group and the nature of acquiring “suicide” data. By definition, studying suicide in vivo always necessarily implies ex post facto data acquisition and analysis. For example, having resting scans on all 12 patients would have given our results added certainty and robustness. Having all BDI scores likewise would have allowed us an objective covariate by which to control for symptom severity. In addition, we were unable to control for season. Our hope was that randomizing the assignment into comparison groups would minimize any variability attributable to season. Finally, due to the severity of their conditions, some of the patients in our experimental groups were scanned while on their medications. Obviously, medications can have an effect on rCBF and, unfortunately, could not be completely controlled for.
To summarize, our comparisons found results consistent with what would be expected in patients with severe depression and impaired impulse control, including significant perfusion deficits in the ventral tegmental area, medial prefrontal cortex (Brodmann’s areas 11 and 25) and general limbic dysregulation among suicide patients. Further research exploring a specific link between low subgenual rCBF as measured by SPECT and suicide in individual patients is warranted.