T he timing of injury to the human brain, in terms of developmental stage, has been a focus of investigation for over 200 years.
1 Knowledge in this area is potentially important in understanding whether there are sensitive periods in development for damage and for repair of neuronal systems. This information could provide clues regarding varied mechanisms active in these neuronal processes at different phases of development.
A dominant theoretical position for many years was the “Kennard effect,” which refers to milder deficits and greater recovery of functions after brain damage early in life.
1 Kennard’s experiments in animal models supported such a conclusion, particularly for the motor system.
2 Other studies of language function after early lesions of the left cerebral hemisphere were pivotal to the argument that the brain is in fact quite capable of functional reorganization.
3,
4A major change has occurred toward support for greater neurocognitive vulnerability of the CNS with earlier damage, especially when this damage is of a diffuse nature (e.g., traumatic brain injury) and very low birth weight.
5 Earlier age at insult has now been linked to greater impairments not only in overall cognitive functioning
6 but also in multiple specific domains of functioning including verbal and written language abilities,
7 attention,
6 and perceptual-motor and spatial skills.
8 Furthermore, closer scrutiny of Kennard’s animal work reveals evidence of more problematic behavioral outcomes after early damage to association cortices that included the prefrontal region.
9Neurocognitive studies of stroke in childhood that provide insight on the influence of age of injury are scarce. Some researchers limited inclusion of subjects to stroke occurring in the prenatal period up to postnatal age of 6 months.
10 Younger age at injury has been related to poorer intellectual function outcomes in children with left-hemisphere lesions.
11,
12 A recent study of children with ischemic stroke found that younger age of stroke (before age 5) was associated with poorer intellectual outcome.
13 Younger age at stroke was associated with parental perceptions of poorer daily living functional outcome after ischemic stroke.
14 A study of executive function in children with “focal” frontal lesions including stroke, bilateral lesions, trauma, and degenerative disorders found that children with prenatal lesions are generally at greatest risk of neurobehavioral deficits.
15 Finally, in contrast to previous relevant language studies,
3,
4,
16,
17 we have provided evidence of a similar pattern of outcome even in the domain of language
18 where children with early lesions had significantly poorer discourse than children with lesions occurring after the age of 1 year.
Apart from our incidental noting that age at focal brain injury was not associated with the rate of poststroke psychiatric disorder,
19 there have been no relevant focal injury psychiatric studies examining age-related plasticity or vulnerability. Studies of children with diffuse CNS injury suggest high rates of psychiatric disorder regardless of whether the injury was congenital or acquired.
20,
21 Furthermore, rates of psychopathology do not appear to be influenced by age at injury
21,
22 except for rare exceptions.
23 However, the association of age at injury and severity of psychiatric disorders has not been studied.
Our goal in this study was to better understand brain plasticity and vulnerability through the study of the relationship of age at the time of brain injury and neurocognitive and psychiatric outcome. The preceding literature review led us to propose the following hypothesis. Children with earlier lesions will have lower scores across neurocognitive domains compared with children with later lesion onset. In the absence of previous reports regarding the effect of age at injury on severity of psychiatric disorders, and corresponding to our first hypothesis, we also hypothesized that earlier insult would be associated with greater severity of psychiatric disorders.
METHODS
Participants
This study was conducted using neurocognitive and psychiatric data that was collected as part of a larger study that also examined family functioning and adaptive functioning in children with strokes.
18,
19,
24 Individuals were included in this study if they experienced a stroke prenatally or during childhood. “Early” lesions were those that occurred prenatally or before the age of 12 months while those classified as “late” were acquired at 12 months or later. This
a priori classification followed that of previous work in the field.
3 In the early stroke group, prenatal onset occurred in 12 children and postnatal onset occurred in five children (day 1 in three children; 2.5 months in one child; 9 months in one child). Age at stroke for the late onset group was 7.8±3.2 years old.
Inclusion criteria for stroke cases were: neuroimaging documentation of a focal, nonrecurrent and nonprogressive supratentorial brain parenchymal lesion caused by stroke before age 14; age 5–19 years old at time of assessment; greater than or equal to 1 year since stroke; and English as first language. The following exclusions were applied: neonatal bleeds (e.g., intraventricular hemorrhages, germinal matrix hemorrhages) potentially associated with prematurity; neonatal watershed infarcts associated with hypoxia; hemoglobinopathies; progressive neurometabolic disorders; Down’s syndrome and other chromosomal abnormalities; malignancy; congenital hydrocephalus; shunts; CNS infections; clotting factor deficiency; stroke in a pregnant minor; transplant status; cerebral cysts; trauma; transient ischemic attack; moyamoya; severe and profound mental retardation; quadriplegia, triplegia, or diplegia; syndromatic vascular malformations (excluding A-V aneurysm ruptures); systemic lupus erythematosis; and multiple lesions (unless in close proximity).
Comparison subjects included children with congenital clubfoot and children with scoliosis who were individually matched to children with stroke according to age of onset of stroke (i.e., early versus late). Matching was also based on gender, ethnicity, socioeconomic status,
25 and age within 1 year. Children were excluded from the comparison group if they had evidence of acquired or congenital CNS injury that may be part of broader syndromes unrelated to the common idiopathic syndromes.
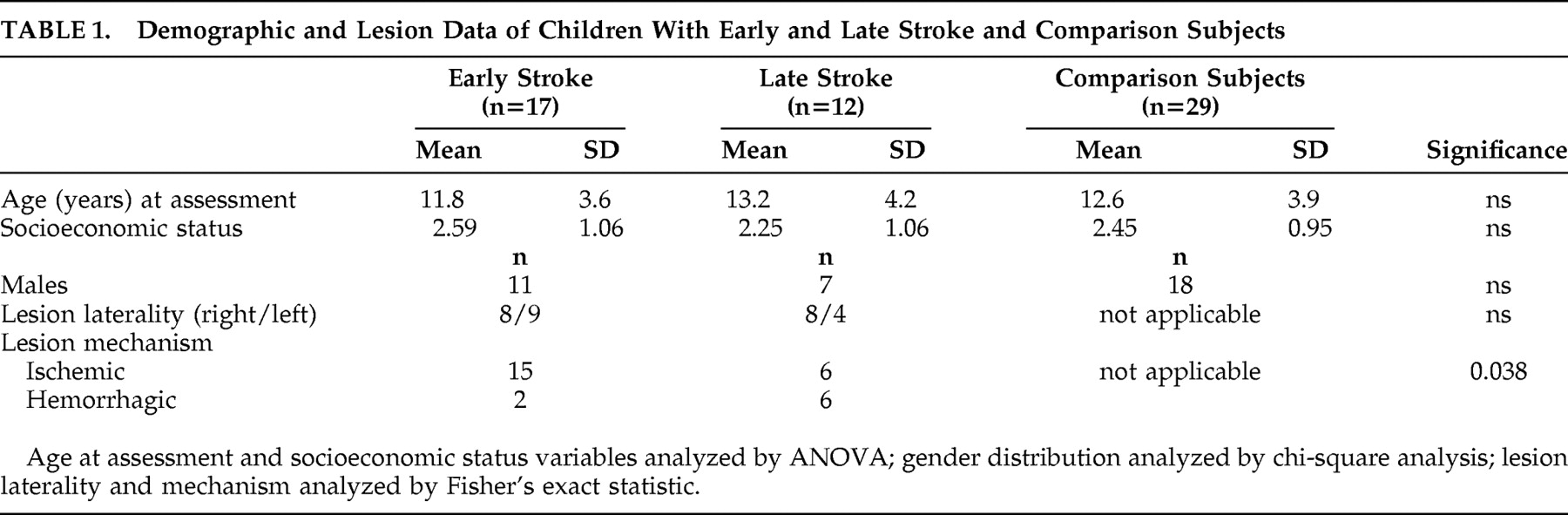
The stroke and orthopedic groups were no different on matching variables of age and socioeconomic status (
Table 1 ). Neither was there an association of age at stroke onset and lesion laterality. However, there was a significant association between early stroke onset and occlusive lesions. There were 27 Caucasians and two biracial children in each of the stroke and orthopedic groups.
Neuroimaging
Protocol MRI scans were obtained (T1-weighted volumetric mode). Twenty-six of 29 stroke subjects underwent research scans. The other three subjects, who could not have a research MRI for technical reasons, had lesion characteristics determined from previous clinical CT scans (two patients) or MRI scan (one patient).
Size of lesions for all stroke participants was calculated according to a standardized protocol which was highly correlated with volumetric analyses conducted on the 26 participants with research MRIs.
26 Lesion size was highly skewed, and therefore early and late stroke groups were compared using Mann-Whitney U. The mean rank for early stroke was 16.6 versus 12.8 for the late stroke group (Mann-Whitney U=75.5, p>0.23).
The study was approved by the institutional review boards at the participating institutions. Adult participants and parents/guardians of minors signed informed consent, and children signed assents prior to testing.
Psychiatric Measures
Schedule for Affective Disorders and Schizophrenia for School-Aged Children, Present and Lifetime Version (K-SADS-PL)
DSM-IV psychiatric diagnoses were derived by conducting the K-SADS-PL semistructured interview.
27 The K-SADS-PL is an integrated parent–child interview which generates diagnoses based on a clinician synthesizing data collected from the parent and child separately, querying present and lifetime symptoms as well as providing data regarding the timing of symptom onset in relation to the stroke or orthopedic diagnosis. Incorporated within the K-SADS-PL is the Children’s Global Assessment Scale (CGAS), which is a clinician-rated measure of overall severity of disturbance.
28 Also administered was the Neuropsychiatric Rating Schedule (NPRS)
29 which is a reliable and valid semistructured interview designed to identify symptoms and subtypes of personality change due to a general medical condition. The outcome variables of interest were CGAS and postmedical condition (stroke, clubfoot, or scoliosis) psychiatric disorder. Children with a premedical condition psychiatric disorder were included in the analyses because they were eligible to develop a psychiatric disorder after their medical condition was diagnosed. Prestroke psychiatric disorder consisted of attention deficit/hyperactivity disorder (ADHD) (one patient), transient tic disorder (one patient), oppositional defiant disorder (two patients), and depressive disorder, not otherwise specified (one patient), and one child had both a social phobia and an anxiety disorder. Preorthopedic psychiatric diagnoses consisted of specific phobia (one patient) and social phobia (one patient).
Fifty-seven of 58 interviews were administered a board-certified child and adolescent psychiatrist (JEM), and all were videotaped. A trained Ph.D. level researcher administered one interview. Eleven interviews were selected randomly to be rated by a second child psychiatrist, to ascertain interrater reliability. The agreement regarding pre- and postmedical condition (stroke, clubfoot, or scoliosis) psychiatric disorder was 11/11 (100%) and was perfect in 9/11 (82%) subjects for specific diagnoses. Interrater agreement for the CGAS was excellent with an intraclass correlation coefficient of .96.
Intelligence Assessment
Wechsler Intelligence Scale for Children—Third Edition (WISC-III), 30
Estimated verbal IQ and performance IQs were computed from two verbal and two performance subtests of the WISC-III (information, similarities, block design, and picture arrangement).
24 A full scale IQ was also computed by averaging the verbal and performance IQ.
Academic Achievement
Wide-Range Achievement Test—Revised (WRAT-R) 31
Achievement was assessed using age-adjusted standard scores for WRAT-R reading, spelling, and arithmetic. The reading subtest requires subjects to recognize letters and single words. The spelling subtest requires copying of simple geometric shapes, name writing, and spelling of single words. The arithmetic subtest requires solving of mathematical problems of increasing complexity.
Language Skills
Multilingual Aphasia Examination (MAE) Sentence Repetition 32
MAE Sentence Repetition was administered to assess verbal working memory. In this test, 14 sentences of increasing length and complexity are read to the participants, and subjects are asked to repeat the sentence immediately after hearing it. In the statistical analysis of this study, the percentile was transformed into a standard score using a standard conversion chart. If a percentile corresponded to more than one standard score (e.g., near the upper and lower tails of the distribution), the highest standard score was assigned.
MAE Token Test 32
The MAE Token Test provided a measure of verbal comprehension and the ability to carry out oral commands. In this test, 20 blocks of varying colors, sizes, and shapes are presented, and participants are given commands of increasing complexity of tasks to carry out with the blocks. The same procedure used in scoring the MAE Sentence Repetition was used to obtain estimated standard scores.
Test of Written Language—Third Edition (TOWL-3) 33
Written language abilities were assessed using the TOWL-3. In this test, participants are asked to spontaneously write a story about a picture presented to them. The three aspects of the story that were scored were contextual convention (spelling, punctuation, capitalization), contextual language (vocabulary, grammar, syntax), and story construction (composition of story, such as plot and organization). These three scaled scores were combined (as described in the manual) to obtain a spontaneous writing quotient standard score.
Visuospatial Skills
Developmental Test of Visual-Motor Integration (VMI) 34
In the VMI, the participant copies a series of geometric figures of increasing complexity. The standard score for this test provided a measure of visuospatial skill.
Memory
California Verbal Learning Test—Children’s Version (CVLT-C) 35
In the CVLT-C, an individual’s ability to learn a list of 15 words in 3 categories (toys, fruit, and clothing) is assessed over 5 learning trials. A distractor list consisting of 15 words is then presented for a single trial and immediately followed by free and category cued recall of the first list. Free, cued recall, and recognition test of the first list are probed after a 20-minute delay. The T score for the total number of words learned on the five learning trials was used as a measure of overall verbal learning ability.
Rey-Osterrieth Complex Figure Test (REY-O) 36, 37
In the REY-O, participants are shown the Rey Complex Figure and asked to copy it while it is still in view. The figure is then removed, and the participants are asked to draw it from memory and then again after a 20-minute delay. For this study, visual memory was assessed by scoring the 20-minute delay figure using the 36 point scoring system, and that raw score was transformed into an age-adjusted T score.
Executive Functioning
Design Fluency 38
Numerous executive functions (cognitive flexibility, creativity, constructional abilities, and working memory) were assessed using the Design Fluency test.
39 In this test, participants are asked to make up as many different drawings as possible which are not real objects, geometric figures, or scribbles. In the free condition, participants were asked to draw as many figures as possible in 3 minutes, and in the fixed four-line condition, participants are required to construct each figure from four lines. The measure used in analysis was the age-adjusted
T score for overall performance.
Multilingual Aphasia Examination Controlled Oral Word Association (COWA) 32
This test of phonemic fluency requires participants to generate as many words as they can that begin with the letters C, F, and L. The executive functions tapped by phonemic fluency are “initiation, simultaneous processing, and systematic retrieval of phonemically similar lexical items.”
40 In this study, standard scores were also obtained in the same manner as described for MAE Sentence Repetition.
Wisconsin Card Sorting Test (WCST) 41
The WCST requires many aspects of executive functioning including cognitive set-shifting, planning, the ability to use feedback to modify behavior, and inhibiting impulsive responding.
39 The test consists of four stimulus cards which vary along the dimensions of geometric shape, color, and number. The participant is given a card and is then asked to match that card to one of the four stimulus cards without being informed of the rule (e.g., match on color, shape, or number). After the participant places the card, the examiner provides feedback as to whether the choice was correct or incorrect. The participants are required to use the examiner’s feedback to determine the rule. Once the participant learns the rule, the examiner switches the rule and the participant’s task is to identify the new sorting rule. Performance on this task was measured using age adjusted standard scores for perseverative errors.
Scoring
Because participants in this study ranged in age from 5.92 to 19.92 years old, some of the tests were administered to participants who were younger or older than the test range. The scoring was then done where subjects who were below the test range were scored using the youngest normative age range available, and those subjects who were above the test range were scored using the oldest normative age range available.
24Data Analysis
To minimize the problem of a minority of participants outside the test range of some neurocognitive tests, we capitalized on the individual matching by calculating a difference score for each comparison/stroke match pair. This was accomplished by subtracting the score of the stroke participant from the score of his or her (age, gender, socioeconomic status, ethnicity) match. The procedure effectively limited the sample to 29 matched pairs. We chose to conduct multiple tests (n=15) across multiple domains of function (n=5) because this work is exploratory. Therefore, because of the small sample size and exploratory nature of the work, we analyzed the differences between groups by Cohen’s
d effect size calculations
42 rather than independent sample t tests.
The psychiatric variables were not subject to test age range problems, and therefore univariate ANOVA was used to examine the differences between the early lesion group, the late lesion group, and the comparison group regarding continuous variables. A Tukey’s b post hoc test was used to determine which groups were significantly different from each other using an alpha level =0.05. Children in the early lesion and late lesion groups were further compared using effect size calculations
42 because of the small sample size. The rate of postmedical diagnosis (stroke, clubfoot, or scoliosis) across groups was compared with chi-square analysis.
RESULTS
Neurocognitive Analyses
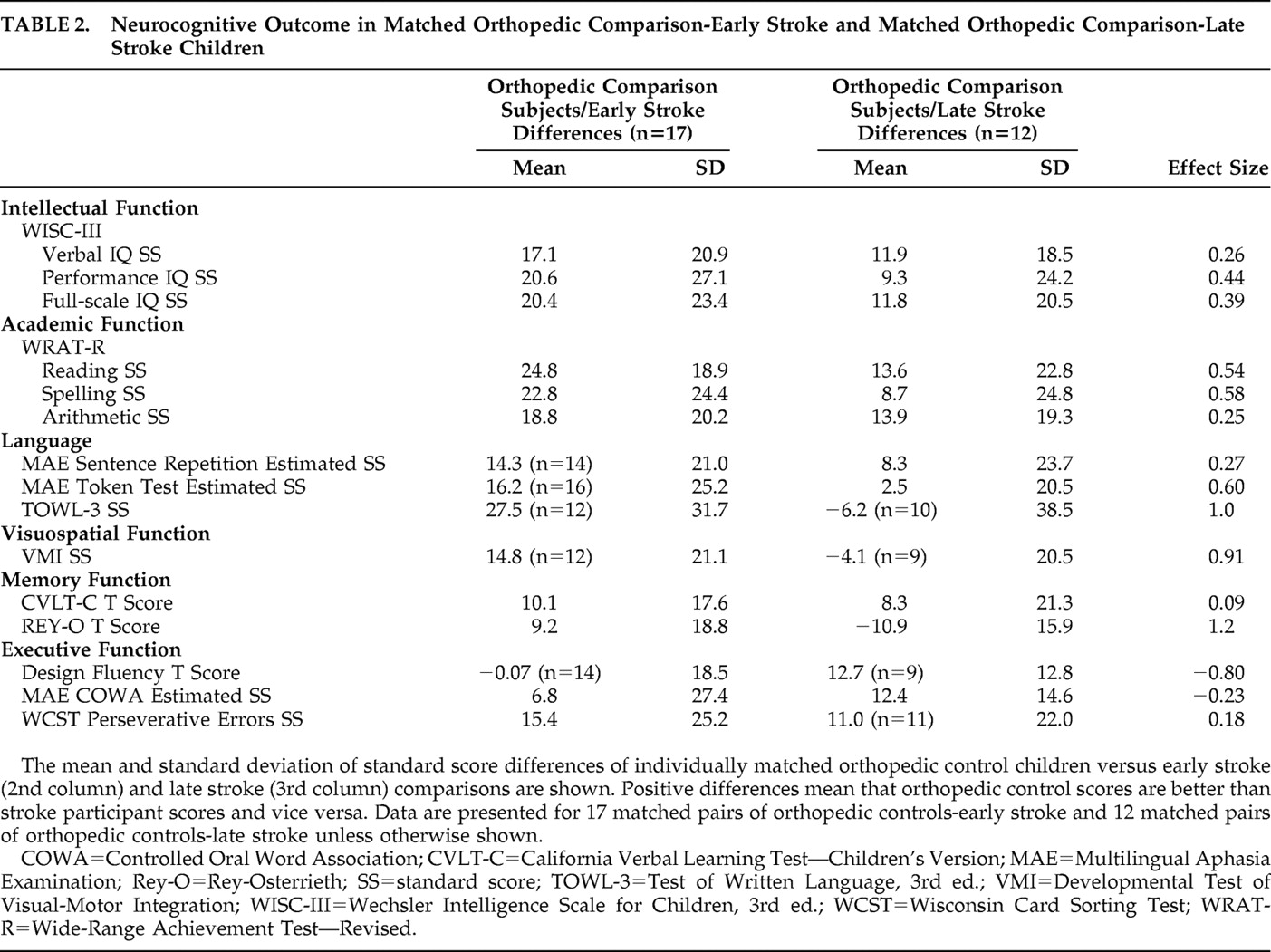
Table 2 shows the effect size analyses of the comparison-early stroke subject pairs versus the comparison-late stroke pairs. Larger differences indicate worse functioning in the stroke participants. The comparison-early stroke differences were larger in 13 of 15 of the tests conducted as follows: The effect sizes were large (≥0.80) in three tests (TOWL-3, VMI, REY-O) spanning three domains (language, visuospatial function, memory). The effect sizes were medium (0.50–0.79) in three tests (WRAT-R reading, WRAT-R spelling, MAE Token Test) spanning two domains (academic function, language). The effect sizes were small (0.20–0.49) in five tests (WISC-III verbal IQ, performance IQ, and full-scale IQ; WRAT-R arithmetic; MAE Sentence Repetition) spanning three domains (intellectual function, academic function, language). The effect sizes were trivial (<0.20) in two tests (CVLT-C, WCST) spanning two domains (memory, executive function). The comparison-late stroke differences were larger in two of the 15 tests conducted as follows: The effect size was large in the Design Fluency test (executive function) and small in the MAE COWA test (executive function). In an attempt to assess the relative influence of the confounded predictor variables, i.e., lesion mechanism (occlusive versus hemorrhagic) and study group (early stroke versus later stroke) on comparison-stroke difference scores, we conducted bivariate correlations. We found that the correlations between study group and comparison-stroke difference scores were larger than the correlations between lesion mechanism and comparison-stroke difference scores in 9/15 of the analyses.
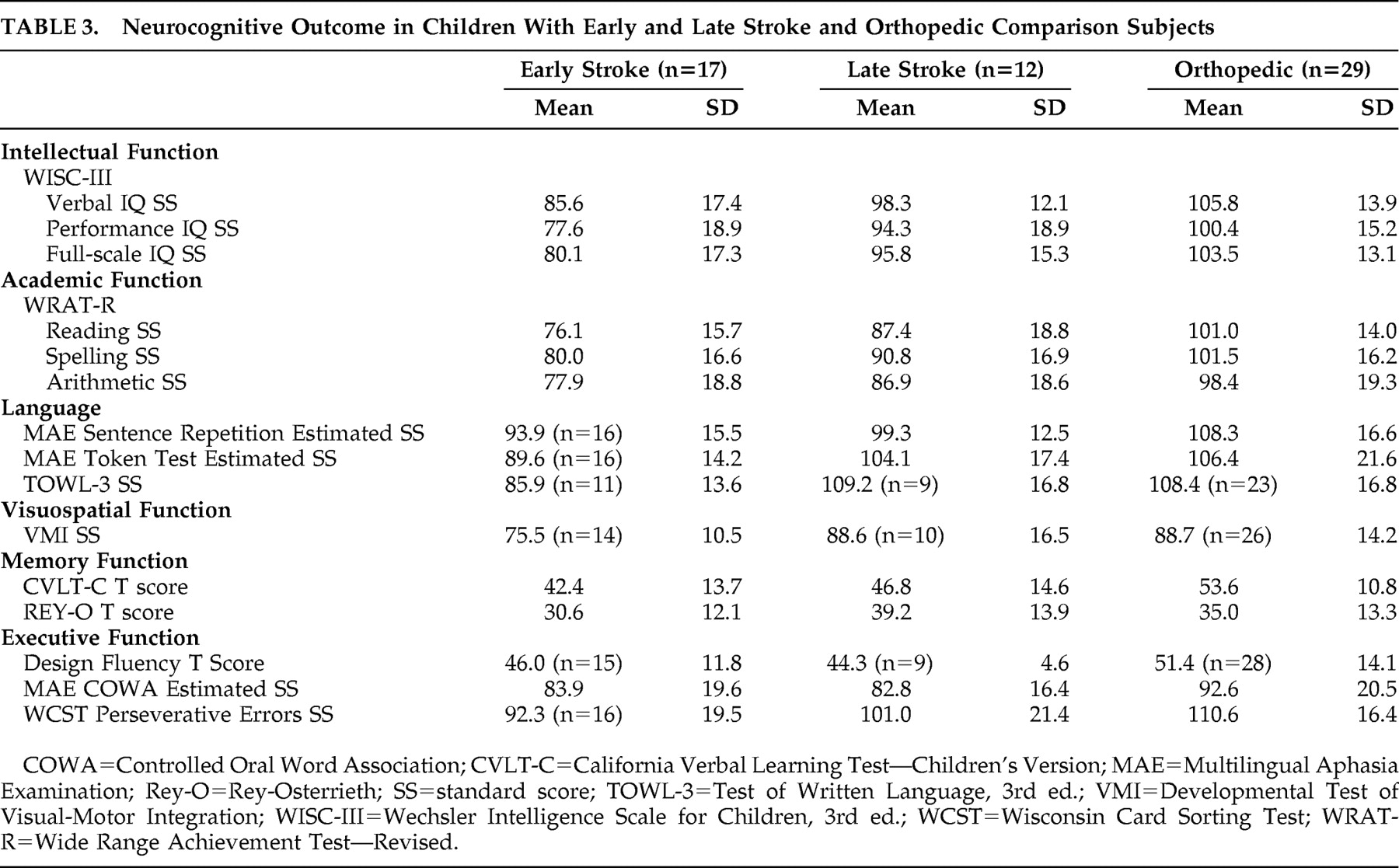
Table 3 shows the group means and standard deviations for the early stroke, late stroke, and orthopedic groups. Group differences were not analyzed because of the problem of a minority of participants outside the test range of some neurocognitive tests.
Psychiatric Analyses
As reported previously,
19 the distribution of postmedical diagnosis (early stroke, late stroke, orthopedic) psychiatric disorder was significant (χ
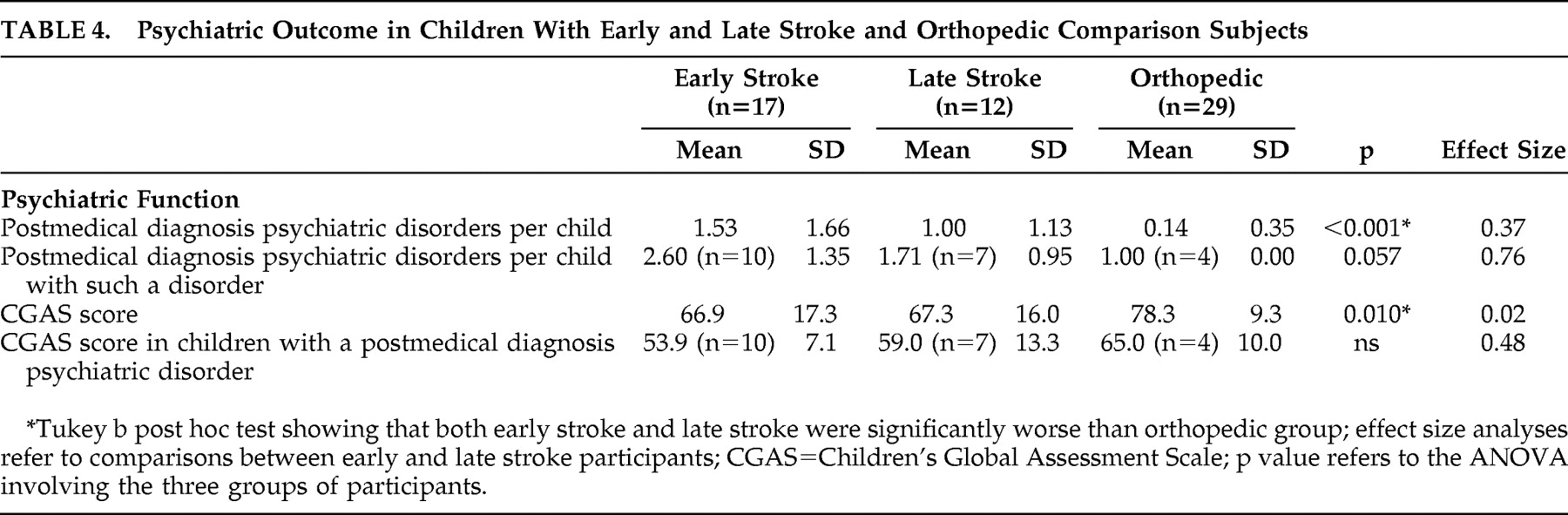
2 =12.6, df=2, p=0.002), occurring in 10/17 (59%) of early stroke patients, 7/12 (58%) of late stroke patients and 4/29 (14%) of orthopedic comparison subjects. The rate of psychiatric disorder was not significantly different between the early-stroke group and the late-stroke group.
Table 4 demonstrates that the mean number of postmedical disorder psychiatric diagnoses was significantly higher in both stroke groups compared with the orthopedic group. The early stroke group had a higher mean number of psychiatric diagnoses than the late stroke group (small effect size =0.37). The mean number of poststroke psychiatric disorders per affected child in the early stroke group was higher than that of the late stroke group (medium effect size =0.76). The CGAS score of the orthopedic group was significantly higher than both stroke groups, which in turn were not significantly different from each other. Finally, the CGAS scores in children affected by a poststroke psychiatric disorder were lower in the early stroke group compared with the later stroke group (small effect size =0.48). In an attempt to assess the relative influence of the confounded predictor variables, i.e., lesion mechanism (occlusive versus hemorrhagic versus control) and study group (early stroke versus later stroke versus control), we conducted regression analyses for each of the psychiatric outcomes. Study group predicted outcome at a significant or trend level for all analyses, while there was no trend for lesion mechanism in any analysis.
DISCUSSION
The major finding in the current study is that stroke-onset prenatally or up until age 1 year is associated with poorer performance (small to large effect sizes) on a wide variety of psychiatric and neurocognitive measures spanning multiple domains of functioning compared with children with later-onset stroke. These include the number and severity of psychiatric disorders in those affected by psychiatric disorder, as well as the domains of intellectual function, language, visual memory, verbal memory, visuospatial function, and academic function. The notable exceptions to this expected pattern were executive function tests which showed that children with late-onset stroke had poorer performance in a Design Fluency test (large effect size) and a verbal fluency test (small effect size).
The predominant finding of early vulnerability is meaningful as it cannot be considered an isolated finding in a particular neurocognitive domain. The unanticipated finding of later vulnerability for selected executive function deficits is also meaningful in that there appears to be differential outcomes in different domains. Taken together, these findings argue against the likelihood that the early stroke group is a nonspecifically or globally impaired cohort. Furthermore, the findings are unlikely to depend entirely on general intellectual function deficits which showed small effect sizes, while various measures of academic function, language function, and visuospatial function showed moderate to large effect sizes. However, these findings were consistent with a previously described nonlinear relationship of executive function and age at injury in children with focal frontal lesions including bilateral lesions, traumatic contusions, penetrating injuries, cerebral dysplasia, demyelinating disorder, and stroke.
15 These findings remain to be replicated and investigated further with a larger sample and tasks measuring a broader range of executive functions.
The psychiatric and neurocognitive findings reported here suggest that even in the context of focal brain lesions, vulnerability rather than plasticity is characteristic after early damage compared with later damage in childhood. This pattern of damage and recovery has been documented in the presence of different types of diffuse brain damage.
5 The same pattern has also been demonstrated in investigations on populations of children with focal brain lesions with respect to intellectual outcome,
11 –
13 daily living functional outcome,
14 and even language function.
18 This pattern of psychiatric morbidity had never been studied in a focal lesion pediatric population.
There are several theories that attempt to explain the findings of vulnerability after earlier lesions. First, from a pathophysiological perspective, the maturing cortical and subcortical neural networks that subserve rapidly developing skills may be especially vulnerable to ischemic damage in clinical and experimental studies and less sensitive to the neuroprotective effects of “preconditioning” in experimental studies.
43,
44 Second, a related neurobiologically based theory is that the protracted course of myelinization increases vulnerability especially in the frontal lobes.
45 Third, early focal brain damage may have implications for neuronal repair including the production of anomalous neuronal reconnections especially if this damage occurred during axonal weeding, because axonal collaterals that would have normally been destroyed are retained.
46 A similar theory also argues that coincident with early damage is a state of the presence of few astrocytes and an active process of neural migration. According to this theory, earlier injuries may in fact disrupt the migratory pattern of neurons in the injured hemisphere and therefore lead to poorer outcomes.
47Neurodevelopmental mechanisms have also been proposed to account for the differential behavioral outcomes of early versus later brain insults in children. One possibility is that development following brain insult is disrupted by a brain lesion early in childhood.
48 For example, the damage may disrupt acquisition of skills and knowledge such that the discrepancy of functioning between the child with a lesion versus the child without a lesion increases cumulatively over time.
49 A component of the increasing deficits over time may be attributed to the inability of the young child to interact normally with the environment due to a brain injury at the ages when these interactions are crucial to the child’s development.
11 Furthermore, skills undergoing rapid and active development at the time of the insult may be more susceptible to disruption than previously established abilities.
6,
7 Certain domains of functioning may manifest aberrant function only at a later point of development when the particular skills subserved by the earlier damaged area are expected to become operational.
50We acknowledge several limitations in this study. First, the small sample size resulted in the necessity to use effect sizes to quantify differences between stroke groups. However, this sample size is not unusual in studies of the relatively uncommon population of children with focal lesions. A second limitation is the broad age range of the sample. The implications of this include differences in brain development for children of different ages and the need to modify scoring for children outside of the age range on some tests. However, the procedure to calculate the difference in test scores between each stroke participant and his or her individually matched control minimized this issue. Our future studies will use tests in which all children are within validated age ranges. Third, the sample was heterogeneous with regard to stroke mechanism (ischemic versus hemorrhagic), as well as laterality and lesion size. However, great care was taken with inclusion and exclusion criteria to obtain a relatively homogeneous sample. Fourth, there is a possibility that the rates of postmedical diagnosis psychiatric disorder would be elevated in the early stroke group relative to the late stroke group because psychiatric disorders are not likely to present before age 1. However, this should not affect the associated level of functioning (CGAS) in individuals who develop psychiatric disorders. Fifth, the ethnic distribution was narrow and may limit generalizability.
The findings of this study require replication in a larger sample of children with focal lesions. The relatively low prevalence of children with focal lesions will necessitate collaboration across multiple sites. Age of onset of stroke should have a wide range from prenatal onset through adolescence and could be treated as a continuous variable. A wide age of onset distribution of brain injury may permit the evaluation of whether there are age windows which elevate vulnerability or plasticity in certain domains of neurocognitive function and specific psychiatric disorders, and if so, what the thresholds of those windows seem to be.
44 A large enough sample would also permit a more refined multivariate assessment of neurocognitive and psychiatric outcome relative to gender, socioeconomic status, lesion characteristics (size, laterality, and location), mechanism of stroke, and time since stroke.
Acknowledgments
This work was completed at the Department of Psychiatry, University of California, San Diego. This study was supported in part by a NARSAD Young Investigator Award (Dr. Max) and a Career Development Award (NIMH K-08MH01800) (Dr. Max). We acknowledge: Katherine Mathews, M.D., for help with selection and recruitment of stroke participants; Fred Dietz, M.D., Scott Mubarak, M.D., and Peter Newton, M.D., for selection and recruitment of orthopedic controls; Brigitte Robertson, M.D., for psychiatric assessment interrater reliability studies; Peter Fox, M.D., and Jack Lancaster, Ph.D., for image analysis; Amy Lansing, Ph.D., for database management; Don Slymen, Ph.D., for statistical consultation; and Damien Ihrig for data collection.