N euroimaging studies of adults with bipolar disorder have implicated a number of limbic and paralimbic regions in the pathophysiology of this condition.
1 Primary candidate regions have included the hippocampus, caudate, putamen, thalamus, and amygdala.
2 The hippocampus is one region in the brain that has received significant attention in mood disorder research, and the highly plastic, stress sensitive hippocampal complex may play a central role in mood disorder.
3 Interest in hippocampal volume stems from neuropsychological and neuropathological studies that implicate this structure in the pathophysiology of bipolar disorders. For instance, enlarged right hippocampal volume is reportedly associated with poor neuropsychological functioning in bipolar disorder,
4 and findings from a neuropathological study have indicated a reduction and dysgenesis of various neuronal cell lines in entorhinal and hippocampal cortex of bipolar subjects.
5 Results of the published MRI volumetric studies of hippocampus in bipolar disorder have been inconsistent.
1,
6 While one study found a trend for decreased volume in the right hippocampus,
7 five studies did not find any changes,
8 –
12 and three studies showed an increased hippocampal size.
4,
13,
14 Some potential causes for hippocampal volume changes include genetic vulnerability,
1 aberrant neurodevelopment process,
15 number of episodes or illness duration,
16,
17 disease subtype,
18 and medication effects.
13,
19 The confounding effects of sex
7,
20 have been considered as well. Given the inconsistency in the literature, we concluded that there was a need for further volumetric studies of the hippocampus in bipolar disorder using high resolution MRI, in which both confounding factors and determinants of volumetric differences can be examined.
METHODS
Subjects
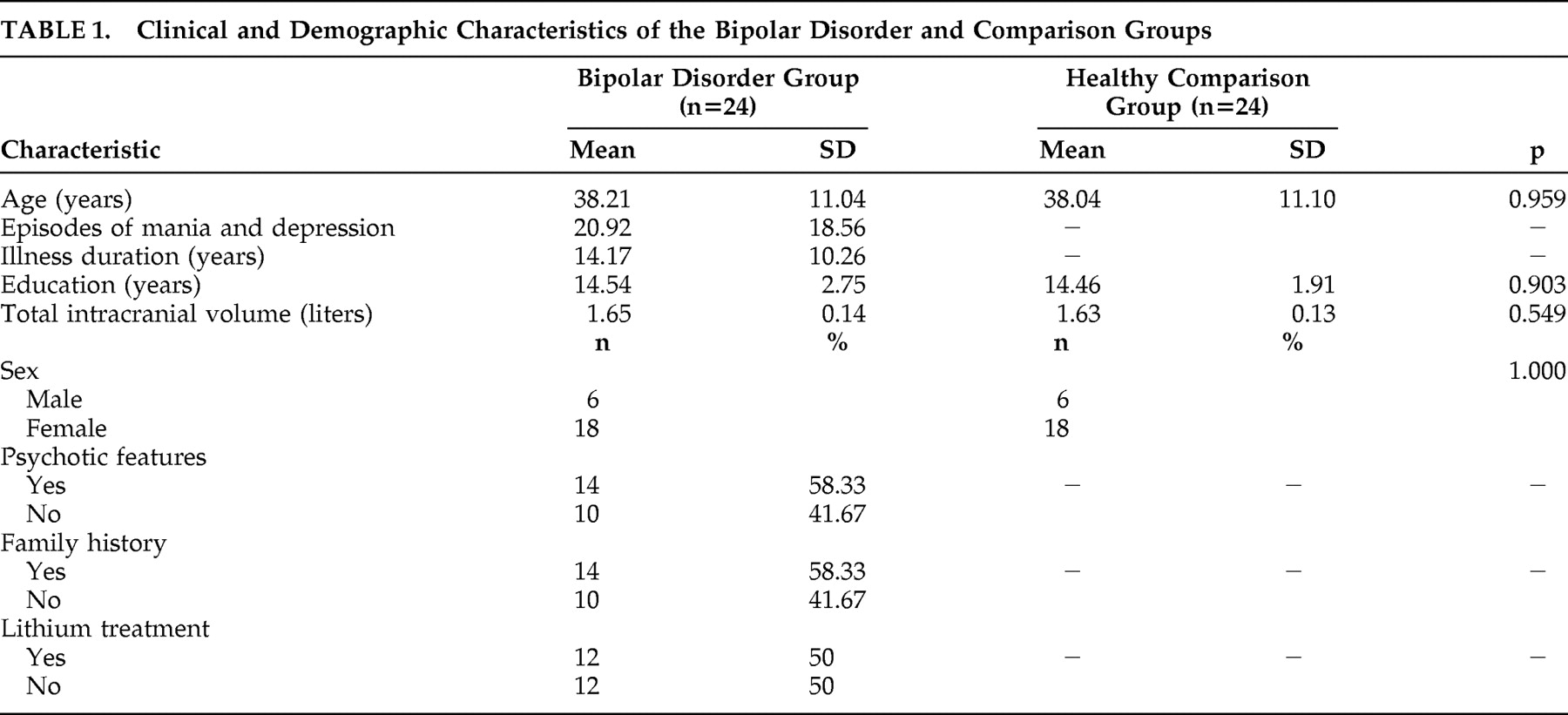
Eighteen women and six men who were 19–59 years old (mean 38.2±11.0 years) with DSM-IV bipolar disorder I were recruited from the Sydney, Australia, Bipolar Disorder Clinic, which is a secondary and tertiary referral service. Research psychiatrists made the diagnoses using the Structured Clinical Interview for DSM-IV (SCID-P) supplemented by a case note review.
21 Participants were considered to meet criteria for bipolar disorder with psychosis if hallucinations and/or delusions were present during at least one affective episode. Subjects were excluded if they had a history of ongoing substance misuse, neurological disease or closed-head injury or had an additional axis I or axis II DSM-IV diagnosis, a medical disorder currently necessitating treatment.
At the time of MRI scanning, 12 participants were taking lithium (mean daily dose 975.1±213.2 mg) with a mean plasma level of 0.77±0.1 mmol/liter, with four of these also receiving sodium valproate. Eight patients were taking valproate, carbamazepine, or a combination of the two, and the remaining four patients were not on medication. Fourteen patients had at least one first-degree relative with an affective disorder, and 10 patients had no family history of affective illnesses.
Patients were compared with volunteers matched for age (within 2 years), sex, handedness (all right-handed), and years of education (mean 14.8±2.1 years) who were recruited by local advertisement. Comparison subjects were screened for a history of neurological or psychiatric disorder (with SCID-NP) or a family history of the same problems. They underwent the same clinical assessments and self-report questionnaires as patients immediately prior to scanning. The Prince of Wales Hospital and University of New South Wales research ethics committees approved the study, and all participants provided written informed consent.
MRI
Imaging was conducted with a 1.5 Tesla GE scanner (GE Medical Systems, Milwaukee, Wisconsin) for T1-weighted 3D structural. A 2D scout mid-sagittal cut for AC-PC plane alignment was first acquired. Then 3D structural MRI was acquired in coronal orientation using a T1-weighted FSPGR sequence (TR/TE=12.2/5.3; flip angle =250; matrix size =256×256; FOV =250×250 mm; no gap between), yielding coronal slices 1.6 mm thick with an in-plane spatial resolution of 0.976562×0.976562 mm/pixel.
Volume Measurement
Hippocampus measurements were carried out by one trained rater (AJ) who was blinded to the subjects’ sex, age, and diagnosis. The periphery of the region of interest on coronal T1-weighted slices was manually outlined using 6.1 Analyze software (Imaging Resource Center, Mayo Clinic, Rochester, Minnesota). The boundaries of the regions of interest were outlined by a cordless infrared light-driven cursor, and the number of voxels within the regions was calculated to produce a total volume for the region of interest. The outlining of the structures always proceeded from anterior to posterior. The hippocampus included the dentate gyrus, the hippocampus proper, and the subicular complex.
22 The protocol used was similar to the one published by Watson et al.
23 Interrater reliability of hippocampal measurement determined on 5 scans (intraclass correlation coefficient [ICC]=0.90) and intrarater reliability on nine scans (ICC=0.98) were both high.
Data Analyses
Left and right hippocampal volumes were normalized to the total intracranial volume of the comparison group using the following formula
23 –
24 :
normalized (or corrected) volume=R × raw volume, where R is the mean total intracranial volume of the comparison subject divided by the patient’s total intracranial volume. Total intracranial volume was calculated by segmenting raw structural images into gray matter, white matter, and CSF using the template in SPM5 software (Welcome Department of Cognitive Neurology, Queen Sq., London).
Normalized hippocampus volumes were compared between patients and comparison subjects, using age, sex, and education as covariates in the analysis. Exploratory analyses were performed to determine the effects of duration of illness, frequency of episodes, disease subtype, family history of affective disorder, and lithium on hippocampus volumes. Bipolar patients and age- and sex-matched comparison subjects were compared on demographic variables. Pair-wise t test was used to compare the difference of raw and normalized volumes of hippocampus between bipolar patients and matched comparison subjects. Hippocampus asymmetry was investigated by comparing right-to-left ratio of volumes between bipolar patients and comparison subjects using pair-wise t test. Effects of clinical features of bipolar disorder on hippocampus volumes were determined by a two-step approach. First, pair-wise t tests were applied to examine the effects of psychosis, family history, use of lithium, episodes of depression, and duration of illness on the normalized hippocampus volumes between bipolar patients and matched comparison subjects. Second, volumetric effects on normalized hippocampus volumes of clinical variables were examined further within the group of bipolar patients by independent t test for categorical variables and Spearman’s correlation analyses for continuous variables. The effects of variables showing significance in unvaried analyses was examined further using ANCOVA to control for effects of age and sex. All statistical analyses were performed using an SPSS software (14.0 for Windows) package. A p value of 0.05 was used as the level of significance for all tests.
DISCUSSION
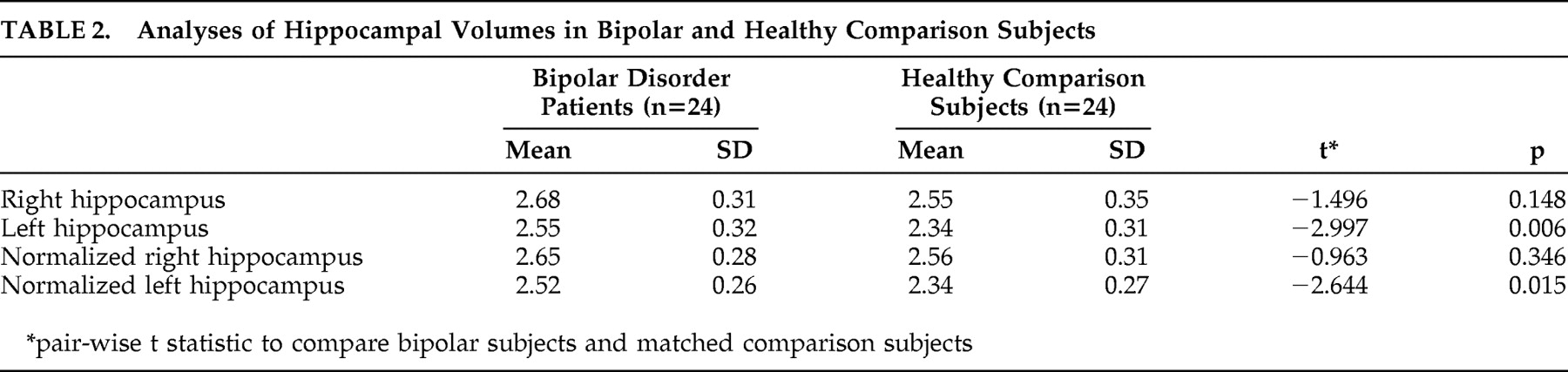
The main finding of this study is that bipolar disorder patients had larger left hippocampus volumes compared to age-, sex-, and education-matched comparison subjects.
Various studies have reported structural brain abnormalities in patients with mood disorder. However, abnormalities in brain regions have been inconsistent. Reduction in prefrontal and frontal volumes was more consistently revealed in both unipolar depression and bipolar disorder, whereas abnormalities in subcortical structures have been different between these two classes of affective disorders. The hippocampus is one of the most studied structures in unipolar depression. Several meta-analyses have confirmed that patients with unipolar depression have smaller hippocampus volumes than healthy individuals.
25,
26 In the unipolar-depression literature, increased illness duration and increased frequency of episodes have been associated with decreased hippocampal volume.
27,
28 Presently, studies of hippocampal volumes in depression have been somewhat inconsistent. This inconsistency may stem from heterogeneity of etiological and associated factors of depression.
The published literature suggests that hippocampal volume abnormalities are common in bipolar disorder, but whether this is due to a reduction or an enlargement is controversial.
4,
7 –
15 Our finding is consistent with the three earlier studies that found increased hippocampal volumes in bipolar disorder
4,
13,
14 but is in contrast to other studies that found no differences between bipolar patients and comparison subjects
8 –
12 or a reduction in hippocampal volumes in bipolar patients.
20A number of potential causes of hippocampal volume changes have been postulated. These include genetic predisposition,
1 neurodevelopment defect,
15,
20 sex,
7,
20 age,
13 duration of illness, number of episodes,
16,
17 disease subtype,
18 and medication effect.
13,
19 A key argument here is whether the abnormality predates the onset of the disorder or develops secondarily and what role drugs play in its development.
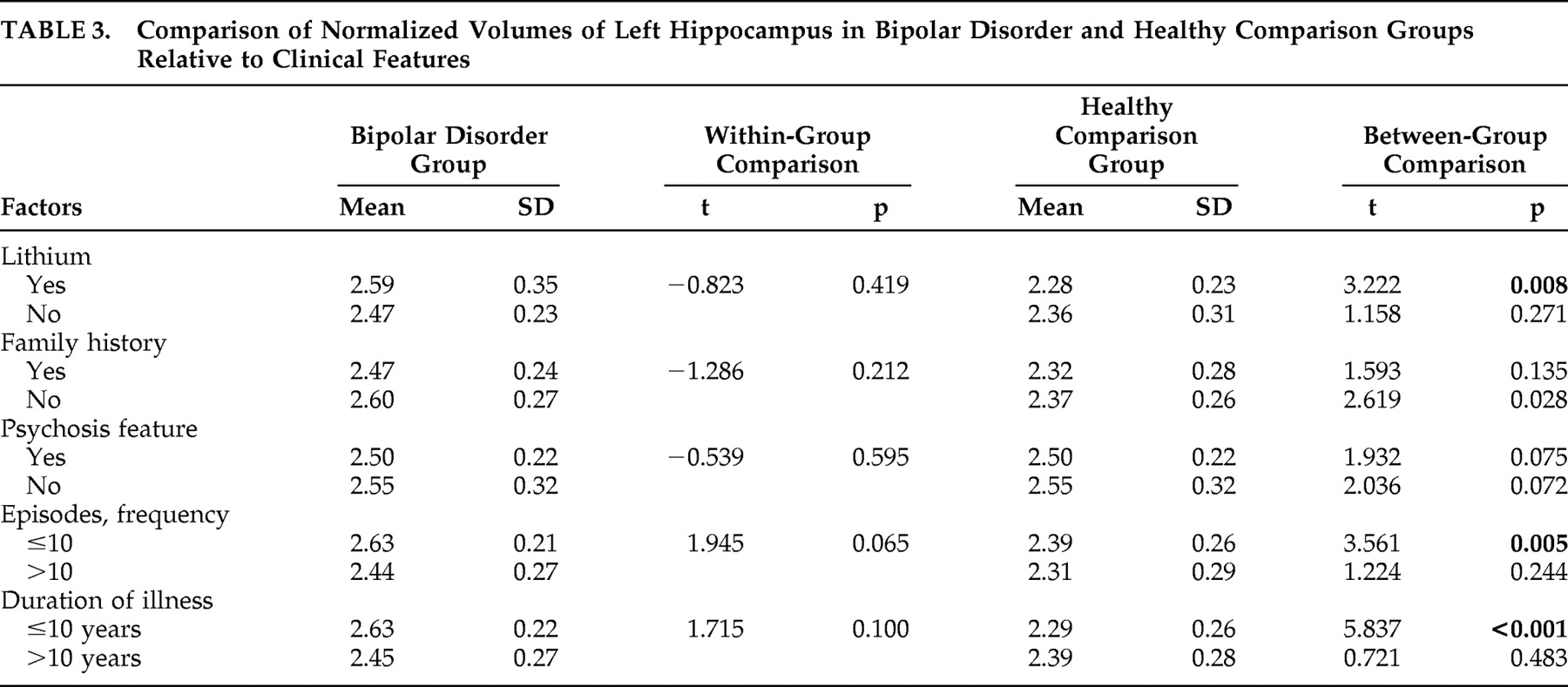
Our finding of a negative relationship between left hippocampal volumes and duration of illness is important in this context. There is some evidence in support of the illness duration and frequency of episodes as being implicated in structural abnormalities observed in mood disorders.
9,
17,
27 –
29 Patients with prolonged course of illness and more frequent episodes have consistently shown poor neuropsychological function.
30,
31 Studies on patients with first-episode affective disorder reported some structural abnormalities at least in a portion of patients, suggesting that these abnormalities are present early in the course of the illness.
15 Ali et al.
17 found that longer duration of illness was associated with larger hippocampal volume in bipolar disorder. Some researchers found that a greater number of manic episodes was associated with a large amygdala volume, a structure anatomically associated with the hippocampal complex.
9 One investigation on pediatric and adolescent bipolar disorder patients—a population probably presenting the initial course of the disorder—found a hippocampal volume reduction suggestive of aberrant neurodevelopmental defect in this structure.
20 However, in a recent study performed by Velakoulis et al.,
32 hippocampal volumes were normal in a first-episode group of patients with affective psychosis, which is consistent with some studies which failed to detect any association between frequency of affective episodes or duration of illness and the hippocampal volume.
10,
13 The literature is therefore by no means conclusive on this topic. Our data revealed that patients with shorter illness duration and fewer episodes of affective disorder had larger left hippocampus volume relative to healthy comparison subjects, and hippocampus volume was inversely correlated with illness duration and number of episodes. This suggests that hippocampal volume was possibly larger early in the course of the illness and decreased as it progressed. These findings could be equated with the role of a neurodevelopment abnormality early in the course of the disorder and a pathophysiologic process that occurs with illness progression; a longitudinal study is necessary for a definitive determination. We view these findings with caution since a retrospective determination of duration of illness and number of episodes is often prone to error, and our study did not include first-onset cases. Furthermore, our sample was too small to yield sufficient statistical power to exclude effect of confounding factors like age. More studies of first-episode cases that are followed up longitudinally are necessary to address this discrepancy.
Another possible factor that may affect hippocampal volume in bipolar disorder is medication. Effects of medications on regional brain volumes have been found in some psychiatric conditions.
33 –
37 Manji and Duman
35 suggested that medication exposure might cause regional volume changes by alteration in the glial cells and neurons. The hippocampus has been implicated as a site for this cellular plasticity.
38 The influence of medication in brain volume analyses may therefore be underappreciated. In previous studies of hippocampal volume in bipolar patients, only two assessed the effect of lithium.
10,
13 In one study, Strakowaski et al.
10 did not find a correlation between hippocampal volume and use of antipsychotics and mood stabilizers. Beyer et al.
13 reported increased left hippocampus volume in older bipolar patients, correlating it to exposure to lithium. Results of our study showed that bipolar patients exposed to lithium had larger left hippocampal volume relative to comparison subjects, but further univariate analysis comparing lithium-exposed and non-lithium-exposed subjects did not reveal a significant difference. However, insufficient information on the length of exposure was our study limitation. Furthermore, in our sample a substantial number of untreated patients (n=8) were taking other plasticity enhancing medications such as valproate and carbamazepine.
Earlier evidence suggests that bipolar disorder with psychotic and nonpsychotic features may be two different subtypes with dissimilar pathophysiology.
18 Neurophysiological investigations demonstrated a reversal of cerebral asymmetry in bipolar patients with a history of psychosis, suggesting that reversed asymmetry may be a trait marker in psychotic mood disorders.
39 Results of one study found that psychotic bipolar disorder patients, but not nonpsychotic bipolar patients, were associated with increased ventricle volumes and a trend toward smaller left hippocampus volume, concluding that hippocampal volume changes in bipolar disorder patients were not marked and did not clearly distinguish the psychotic bipolar disorder patients and nonpsychotic bipolar patients.
18 Consistent with this, our results did not reveal a significant difference in left hippocampus volume between psychotic bipolar patients and nonpsychotic bipolar patients relative to their comparison subjects.
Genetic predisposition is another putative potential factor for structural abnormalities in bipolar disorder. A volumetric study on twins with bipolar disorder showed a significant decrease in left hemispheric white matter volume in patients and co-twin relative to comparison twin subjects.
40 One study reported decreased hippocampal volume in affected versus unaffected twins and no hippocampal volume changes in unaffected co-twins of bipolar patients.
1 Brambilla et al.
41 noted that family history of mood disorders did not have any significant effects on amygdala volume, a structure consistently reported to be involved in bipolar disorder. In this study, we did not notice any significant difference in the hippocampal volume based on family history. However, studying pediatric bipolar patients at the onset of the illness is likely to be a useful strategy, since children and adolescents with bipolar disorder probably comprise a population with a more genetic susceptibility for the illness and are less likely to have many confounding variables associated with ongoing medication and illness chronicity.
In summary, we found that the left hippocampus was larger in a group of adult bipolar subjects relative to a healthy comparison group. The reason for this is unclear. In our sample, it was not associated with family history, psychotic features, or medication exposure. A negative association was found between left hippocampal volume and number of episodes or duration of illness. Our data suggest that the hippocampus might be larger in the early phase of bipolar disorder but becomes smaller with time. Nevertheless, a further longitudinal study, preferably in first-episode cases, is necessary to determine this conclusively.