M r. A, a right-handed man of Austrian-Argentine descent who was 60 years old at the time of his first visit to our tertiary center, had been referred for evaluation of his chronic treatment-resistant depression. The patient presented with his first major depressive disorder episode at age 39, which was not related to an obvious significant life stressor. Mr. A had a family history of both documented affective disorder and other less well characterized illnesses in his first- and second-degree relatives. Mr. A’s paternal grandfather had spent time in a psychiatric hospital in Europe for unknown reasons but possibly related to his participation in World War I, and his sister had been diagnosed with depression which responded to antidepressants.
Mr. A’s first depressive episode was successfully treated with a tricyclic antidepressant, which was discontinued after 1 year. Mr. A remained symptom-free until he was 49 years old, when a second severe, nonpsychotic major depressive episode developed approximately 4 months after the detection and successful surgical resection of a localized prostate cancer. Symptoms were sustained (although with some fluctuations in severity lasting a few months) with little to no response to various treatments including paroxetine, fluoxetine, sertraline, mirtazapine, and clomipramine prescribed at maximal doses and taken for sufficient duration. Some of these antidepressants were augmented with lithium, divalproex sodium, risperidone, or olanzapine without benefit. Supportive psychotherapy was provided throughout the episode combined with a formal trial of cognitive behavior therapy (CBT), again without clinical benefit.
The patient was referred for consultation to our center after 10 years, at age 60. At the time of first evaluation, the patient had been unemployed for 3 years, was receiving disability benefits, and had experienced a loss of 17 kg in the previous 6 months. Neurological and medical examinations were normal. On mental status examination the patient appeared as a thin 60-year-old male, looking older than his stated age, disheveled, with poor self-care and grooming, no abnormal movements, and coherent but slow and impoverished speech. His thought content was focused on overvalued ideas of financial ruin. The patient was not suicidal. His mood was self-described as “horrible” and his affective expression was flat and congruent with the reported mood. He had no hallucinations in any sensory modality, Schneiderian first-rank symptoms, passivity experiences, obsessions, compulsions, phobias, depersonalization, or derealization. He retained insight into the nature of his health problem and his judgment was appropriate. A new medication regimen was instituted and included extended-release venlafaxine, 225 mg p.o. daily, and quetiapine, 200 mg p.o. twice daily, which remains unchanged to this day. A course of CBT was also initiated, but the patient was unable to comply with therapy demands due to poor attention and low energy.
Given continued severe symptoms, failure of standard and combination pharmacotherapy and psychotherapy, and absence of any neurological contraindications, ECT, a relatively seldom used treatment in his cultural setting, was considered for this patient. The patient underwent a series of 15 bilateral ECT sessions with seizure duration between 28 and 55 seconds. This ECT course brought about a mild and short-lived subjective amelioration; mood, initiative, sleep, and appetite modestly improved. The patient and his family deemed this improvement unsatisfactory.
Mr. A regressed to his previous level of symptom severity approximately 6 weeks after the last ECT session, in spite of continued treatment with venlafaxine, 225 mg per day, and quetiapine, 200 mg twice a day, as stated above. He had subjective complaints of attention and memory difficulties and these worsened transiently after the ECT course. Both the patient and his family felt the mild improvement in symptoms did not warrant a further course of ECT or maintenance ECT sessions. As recommended by a team meeting in the psychiatry department, the patient and his family were then offered deep-brain stimulation (DBS) of white matter immediately adjacent to Brodmann’s cortical area 25 (Cg25-DBS, subgenual cingulum) bilaterally as an experimental treatment for refractory depression that had shown promise in a recently published case series.
1 This procedure was offered instead of other neurosurgical options such as ablations or vagus nerve stimulation because of the extensive experience in our center (i.e., >200 interventions) with the use of DBS as a treatment of idiopathic Parkinson’s disease. Written consent was obtained from the patient and his wife. The protocol and consent form were modified from the original Canadian Cg25-DBS pilot study
1 and explicitly stated that the procedure was experimental, requiring testing of different stimulation parameters to optimize potential clinical effects and to minimize adverse events. Prior to the procedure, the approval by the local bioethics committee, the signed consent form, a summary of the patient’s history, and the technical description of the device were all reviewed—and approved—by the Argentine National Administration on Medications and Medical Devices (ANMAT), the national government agency regulating the local use of experimental drugs and medical devices. The patient was implanted as previously described.
1With the patient awake, different contact patterns were probed in the operating room, and some patterns consistently produced a sense of well-being that the patient described as “a relief of the tension in my neck.” After the operation, additional systematic testing of different pairs of bilateral and symmetrical contacts at varying current settings was performed in the first 3 days while in the hospital (contacts were changed every 3 hours during the daytime). Initial stimulation parameters were selected based on the Canadian pilot study
1,
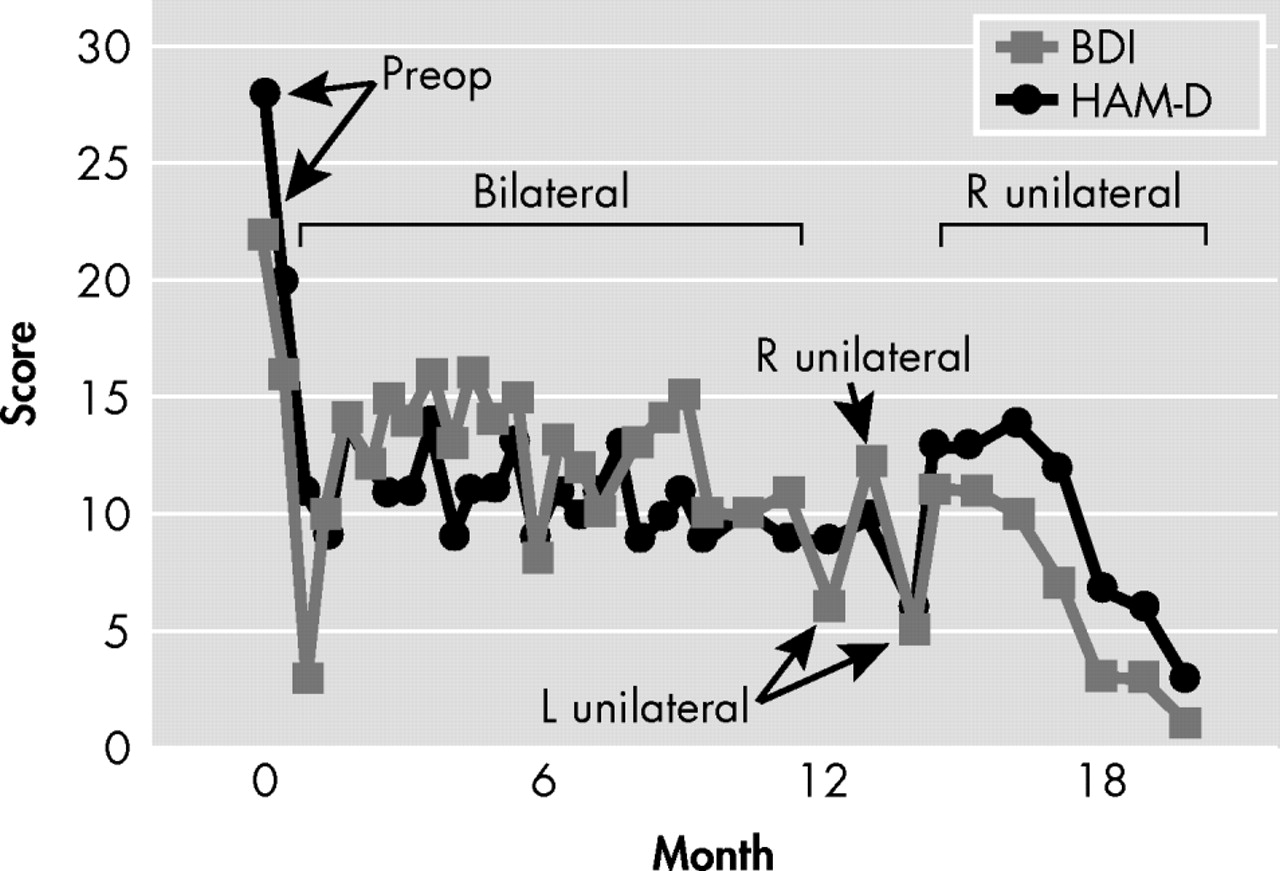
2 (voltage 1.5 V, pulse width 70 μsec, and frequency 90 Hz), and all these parameters were titrated upward to a maximum of 6 V, 90 μsec, and 130 Hz for each pair of electrodes at the aforementioned time. Testing of different combinations of contacts and currents continued in an outpatient setting, with evaluations and changes performed every 2 weeks. Response to each pattern of stimulation was based upon the subjective account offered by the patient while in the hospital, and then by means of the Beck Depression Inventory (BDI) and 21-item Hamilton Depression Scale (HAM-D), as depicted in
Figure 1 . This process continued for 6 months with stimulation parameters in electrodes 2 (left hemisphere) and 6 (right hemisphere) associated with the most consistent symptom amelioration: frequency 120 Hz, pulsewidth 90 μsec, and voltage 4.5 V. After 6 months, the patient’s improvement reached a plateau, despite an initial and sustained drop in both Hamilton and BDI scores (see
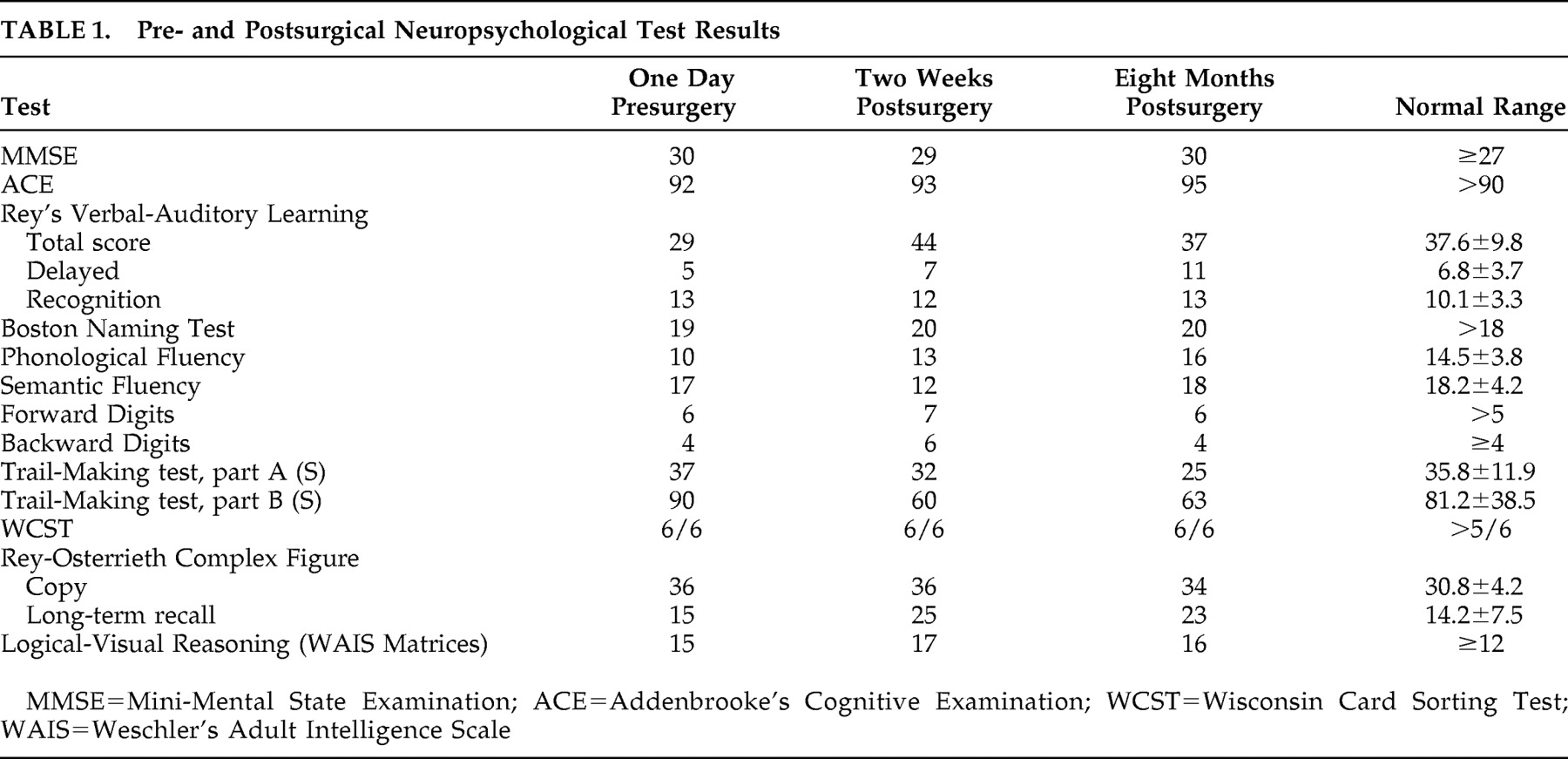
Table 1,
Figure 1 ). Further adjustments did not facilitate additional change in scores, which remained stable. As such, at 12 months and based on literature data suggesting a differential contribution of the left and right hemispheres to mood regulation, we began testing unilateral Cg25-DBS with those stimulation parameters found to be optimal in bilateral stimulation with the purpose of pursuing complete remission of depressive symptomatology. As shown in
Figure 1, left unilateral (L) stimulation appeared to be followed by rapid (i.e., less than 1 week) worsening of mood. At the next appointment (2 weeks later), Mr. A actually stated “by the time I reached the elevators, I was not feeling well already.” Even though, as depicted in
Figure 1, the patient did not return to his preoperative degree of depressive symptomatology, per his subjective account of mood, feelings of inner tension, and anergia, his status was distinctly different than experienced with bilateral stimulation, beginning within the hour of changing stimulation parameters. At this point, settings were switched to right unilateral stimulation (R,
Figure 1 ), which, in contrast to left unilateral stimulation, resulted in not only reversal of the new symptoms but improvement beyond that seen with bilateral stimulation. With right unilateral stimulation, consistent remission of symptoms was seen within 2 weeks, both as rated by the patient with the BDI and as directly observed by the psychiatrist and quantified with the HAM-D. Mr. A was made aware by staff when stimulation parameters were being changed, but he remained blind to what these parameters were, and if stimulation was bilateral, right, or left. The psychiatrist who conducted the rating was not blind to these changes.
Retesting of left unilateral stimulation performed with the purpose of ruling out a placebo effect or a temporary worsening unrelated to the type of stimulation resulted in similar transient worsening of symptoms within hours (
Figure 1 ) and similar accounts of deterioration in mood, anxiety, and lack of energy, whereas reinstitution of right unilateral stimulation brought about full symptom remission within 4 weeks that continues to the present time (now over 12 months later). No washout periods with stimulators off, or resumption of bilateral stimulation, were used when changing unilateral stimulation from one side to the other. No further trials were conducted because the patient remains fully remitted on unilateral right stimulation at 120 Hz, 90 μsec, and 4.5 V.
Overall, the surgical procedure and testing of all contacts at the various parameters was generally well tolerated. There were no behavioral or cognitive adverse events at any settings (
Table 1 ), and unilateral stimulation was not associated with cognitive symptoms. However, initial postoperative testing of the most proximal electrodes (bilateral stimulation, 120 Hz, 90 μsec, 3 V at contacts 3 and 7; i.e., those lying immediately adjacent to subcallosal cingulate gray matter) resulted in no change in mood symptoms but acute onset of orthostatic hypotension that normalized immediately when stimulation was discontinued. Such autonomic changes were seen at no other contacts. Retest of the deepest electrodes bilaterally 6 months postoperatively was performed because prior orthostatism could have been related to the postoperative status. With repeat testing, reversible asymptomatic, albeit significant (−15 mmHg), decrease in mean arterial pressure was induced within 60 seconds of stimulation onset. Unilateral stimulation using these contacts was not performed.
DISCUSSION
Major depressive disorder is the leading cause of disability-adjusted life years lost for people ages 15–44 years old worldwide.
2 Although the etiology of depression remains relatively obscure, the available evidence suggests that major depressive disorder is a complex disorder that results from an interaction of biological, genetic, psychosocial, and environmental factors.
3 Functional neuroimaging studies have broadened our understanding of how these complex factors impact the CNS to produce major depressive disorder symptoms
4,
5 and how various treatments mediate recovery.
6 –
9 Bilateral deep brain stimulation of the white matter immediately beneath the subcallosal cingulate was in fact developed based upon the results of such studies,
1 and is currently being investigated as a potential treatment option for patients who fail to respond to approved methods for management of treatment-resistant depression.
10A critical issue in the management of this case was the criteria used both to define treatment resistance and as a foundation for offering a potential new treatment of yet unproven efficacy. Treatment-resistant depression has been traditionally defined as an inadequate response to an adequate course of treatment in a patient with major depressive disorder.
11 Opinions vary regarding precisely what constitutes an inadequate response. Thase and Rush
12 proposed a five-stage system for defining treatment-resistant depression, which ranges from lack of response to a single adequate trial of antidepressants to lack of response to ECT, the most effective single treatment for depression.
13,
14 Reported prevalence rates for treatment-resistant depression vary according to which criterion is used to define the condition and the clinical setting in which the study was carried out, with progressively higher rates occurring in ambulatory settings, psychiatric hospitals, and tertiary care settings. Our patient would clearly meet Stage 5 treatment-resistant depression, defined as failure to respond to three antidepressants and failure to respond to ECT.
11 This condition, as exemplified by our patient, is associated with a remarkable burden in direct and indirect costs, in loss of quality of life for patients and their families, and in increased risk for suicide and substance abuse.
15 –
17 While multiple factors are known to contribute to treatment nonresponse among individuals with major depressive disorder, the two most common causes—poor adherence to prescribed treatment
18 and poor tolerability
19,
20 —were not at issue here. Chronicity of depression (in terms of duration of the present episode and number of previous episodes), older age, psychiatric and medical comorbidities, and symptom severity are all associated with delayed time to remission
12,
21,
22 and were serious considerations in this case. It is well recognized that medical and psychiatric comorbidities can play a crucial role in treatment resistance. In STAR*D, 53% of patients had one or more significant medical comorbidities. Older age, lower socioeconomic and educational status, and lack of a family history of depression were additionally associated with this complicating factor
23 and contributed to treatment resistance. In this case, there were no such comorbid factors except for the inactive prostate cancer.
The steps taken in the treatment of Mr. A when his depressive symptoms failed to respond to efficacious treatments reflect the usual practice in the management of treatment-resistant depression, though, as noted above, there is a remarkable paucity of well-controlled and adequately powered studies comparing different treatment options to manage advanced-stage treatment-resistant depression.
11 Increasing antidepressant dose switching or combining with a second class of antidepressant, augmentation strategies (with lithium, T3, or an atypical antipsychotic), and an adequate course of ECT are the usual steps to manage treatment-resistant depression and were tried in succession with Mr. A, albeit unsuccessfully. Mr. A’s case is an example of a significant number of patients who remain seriously ill despite currently available approaches to treatment-resistant depression. A major shortcoming in the treatment of depression in general, but in treatment-resistant depression specifically, is the lack of predictors of response to particular treatments in individual patients.
24 In the last decade, functional brain imaging techniques have begun to delineate the putative brain circuits that are altered in depression, and the functional brain correlates of antidepressant treatment nonresponse.
25 –
27 These research efforts have prompted the design of interventions that attempt direct modulation of abnormal circuits for alleviation of otherwise refractory depressive symptoms.
5Brain Circuits Involved in Depression and Deep Brain Stimulation of Brodmann’s Area 25
A brain circuit model explaining the development, response to treatment, and neurobiological basis of treatment nonresponse in depression maintains the classical neurological tradition of symptom localization, initially using lesion-deficit correlational analyses.
5 Before the advent of widely available functional brain imaging techniques, the study of the effect of discrete brain lesions helped to delineate areas putatively involved in the onset of depressive symptoms. With the help of those studies, the prefrontal cortex (PFC) and different cerebral structures interconnected with it have long been recognized as key brain areas in the pathogenesis of depression. The last decade witnessed a myriad of studies that used functional brain imaging techniques with increasingly better anatomical resolution to define the role of cortical and subcortical brain areas involved in the development, maintenance, response to treatment, and treatment-refractoriness of depressive illness.
5 –
9,
26,
27Concordant with the results of lesion studies, resting-state blood flow and glucose metabolism measures using PET commonly reported frontal cortex and cingulate abnormalities, especially decreased function.
8,
28 Within the frontal lobe, the most consistent abnormalities have been found in ventrolateral and dorsolateral prefrontal cortex (Brodmann’s areas 9, 10, 46, and 47), as well as orbitofrontal and ventromedial PFC (Brodmann’s areas 10, 11, and 32). Less consistent abnormalities have been reported for limbic and subcortical structures, including amygdala, anterior temporal and insular cortices, and sections of the basal ganglia and thalamus.
5 Both bilateral and asymmetrical findings have been reported. Although some functional neuroimaging findings are consistent, others display significant interindividual variability, possibly related to differences in genetic predisposition, early life adverse experiences, heterogeneity in clinical presentation, illness severity, and cognitive dysfunction. A replicated observation in these resting-state studies has been the inverse relationship between PFC activity and depression severity. Brody and coworkers
29 have proposed a more complex relationship between behavioral abnormalities and cerebral activity in depression, in the form of a ventral-dorsal segregation of PFC functions. In this view, anxiety/tension is positively correlated with ventral PFC activity, and psychomotor and cognitive slowing are negatively correlated with dorsolateral PFC activity, findings supported in viewing brain imaging studies that compare major depression and generalized anxiety disorder.
30Functional neuroimaging studies that explore the neurobiological basis of response and refractoriness to diverse treatments for depression are of particular importance in the definition of potential targets for direct modulation of cerebral structures in patients with treatment-resistant depression. Resting-state PET studies and fMRI using behavioral tests have consistently reported that increased pretreatment activity in the pregenual cingulate cortex (aCg24; Brodmann’s area 24) distinguishes responders to a series of antidepressant interventions from nonresponders.
5,
31 –
33 In contrast, subgenual cingulate cortex (Cg25, Brodmann’s area 25) hyperactivity has been shown to predict response to sleep deprivation
7 and cingulotomy
34 in patients who had previously not responded to antidepressants, thus constituting a potential neurobiological marker of treatment-resistant depression.
35Mayberg
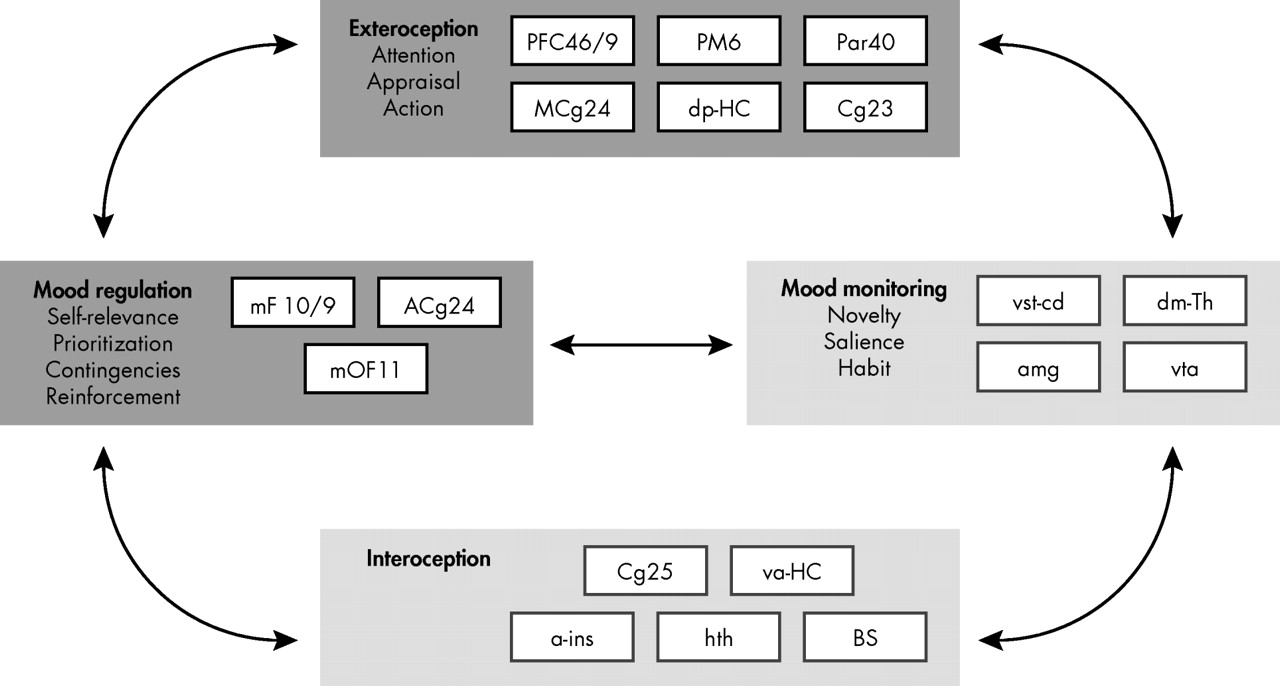
5 recently elaborated on a previously proposed multinode circuit model of clinical depression that evolved mainly from the functional neuroimaging findings summarized above. In this model, regions of potential involvement are grouped in four major nodes with different roles in the onset and maintenance of, but also in response to treatment of, depressive symptoms (
Figure 2 ). In this model, two of the clusters are mostly concerned with interoceptive phenomena and the somatic correlates of depression (
Figure 2, light gray panels), and the other two clusters are concerned with exteroception and neocortical processing and their abnormalities during depressive episodes (
Figure 2, dark gray panels). As consistently observed across studies, commencing with early functional neuroimaging reports, reciprocal interactions between ventral limbic—“interoceptive”—and dorsal neocortical—“exteroceptive”—regions are characterized by relative hyperactivity of the former and relative hypoactivity in the latter.
5 Medial PFC, including orbitofrontal structures, are considered separately in the model to account for their distinctive role in the cognitive control of emotional status. Similarly, subcortical structures participating in cortical-limbic loops involved in emotion, including amygdaloid nuclei, ventral tegemental area, thalamic nuclei, and ventral striatal regions, have been segregated from anterior insula and especially the hypothalamus, given their role in the processing of novel stimuli and salience as well as in reinforcement and therefore learning.
5,
36 –
40 The proposed model provides a conceptual framework to understand the rationale for using a series of DBS targets in depression. Thus, in addition to Cg25, other targets modulating cortical-limbic circuits presumably abnormal in depression have been proposed, including anterior limb of the internal capsule, nucleus accumbens, inferior thalamic peduncle, rostral cingulate gyrus, and left forebrain activation via vagus nerve stimulation.
10,
39 –
43 However, the model does not incorporate emerging data on an asymmetrical role of brain hemispheres in mood regulation, as illustrated by the case of Mr. A.
Asymmetrical Contribution of Brain Structures to the Development of Depressive Symptoms and Response to Treatment
The asymmetrical response of Mr. A to subcallosal cingulate DBS suggests a differential contribution of left and right prosencephalic structures in mood regulation. Structural and functional asymmetry of the human brain has been recognized since the second half of the 19th century due to the pioneering work of Dax,
44 Broca,
45 and Wernicke
46 on the preeminent role of left-sided lesions in the production of most cases of aphasia. An additional landmark in the study of brain asymmetry was the work of Tseng and Sperry
47 and their collaborators with patients who had undergone surgical transection of their corpus callosum to prevent the propagation of seizures. This work extended the concept of brain asymmetry to “higher” cognitive functions beyond language. Studies on hemi-neglect in patients with right-sided lesions of the brain revealed a significant asymmetry in the neural circuits involved in the regulation of attention to outside events, which involves not only a series of sensory modalities but motivational and emotional responses to them as well.
48 However, knowledge of the brain’s functional asymmetries and their impact on emotion and behavior is limited, in part, due to the fact that good experimental models have been lacking until recently. This is likely, in part, due to the view that lateralization of brain functions and behavior has long been considered a purely human trait.
49 The seminal work of Nottebohm and colleagues
50,
51 on asymmetries in the neural control of song production in birds has modified this perception. Subsequently, asymmetric contributions of brain hemispheres to a variety of behaviors have been recognized as a ubiquitous feature of CNS organization across species, including fish, birds, amphibians, rodents, and nonhuman primates,
52 though themes relative to “higher” cognitive functioning have been more intensively studied than asymmetric contributions of brain hemispheres to emotion, autonomic/visceral sensation, and mood and motivated behavior.
Nonetheless, hemispheric laterality has been a preeminent theme in the field of early lesion correlation with depression (i.e., whether left and right cerebral structures contribute differently to dysregulation of mood and the emergence of depressive symptoms). A series of studies supports the hypothesis of an asymmetrical role of the two hemispheres. The majority of data showing a differential role of left versus right CNS circuits in the generation of depressive symptoms were derived from studies investigating incident depression after a localized cerebral lesion, especially a stroke.
53 –
56 These studies offered evidence that left (but not right) anterior hemisphere lesions were causally associated with depression, which was interpreted as suggesting that dysfunction of left prefrontal circuits might be a feature in this disorder.
An alternative explanation, in light of more recent studies on brain function as described below, could be that right PFC activity might become predominant after suffering left-sided lesions, and this imbalance instead of left PFC dysfunction might underlie the development of depressive symptoms. Early positron emission tomography (PET) and electroencephalographic (EEG) studies suggested a pronounced left PFC hypoactivity
57,
58 as well. However, a series of attempts at replication, as well as meta-analyses, do not support a systematic effect of lesion location on poststroke depression.
59 –
61 Koenigs and Grafman
62 recently reported on the association between left, right, or bilateral cerebral lesions and depressive symptoms 15 and 35 years after the index brain injury in a cohort of male Vietnam War veterans. The demographic homogeneity of the sample permitted excellent comparability between groups and in addition with a group of veterans who had not sustained significant brain damage. The study concluded that in this population in particular there were no associations between depressive symptoms and side of the cerebral lesion.
62 Taken together, studies on the correlation between discrete brain lesions and depression have not clearly demonstrated a uniform role of hemispheric laterality in the appearance of depressive symptoms.
Findings of altered bilateral activity of components of circuits involved in depression have been observed, but other studies strongly suggest an asymmetry in the contribution of anterior cortical-limbic circuits to the appearance of depressive symptoms. Dougherty et al.
34 attempted to define potential resting-state cerebral activity predictors of response to cingulotomy, using [
18 F]fluorodeoxyglucose (FDG)-PET. The study is of particular interest because the patients belonged, like Mr. A, to a population with the most severe and most treatment-refractory forms of major depressive disorder. They observed that hyperactivity in the left subgenual cerebral cortex and left thalamus correlated with improvement in depressive symptom severity following cingulotomy.
34 In a study exploring the relationship between changes in cerebral metabolic activity and improvement of depressive symptoms after sleep deprivation, Wu et al.
7 observed that improvement in depressive symptomatology was correlated with an increase in the activity of the left temporal cortex and a decrease in left anterior medial PFC, without similar contralateral findings. In a recent study, Konarsky et al.
27 detected an increase in glucose metabolism in Brodmann’s areas 24 and 32, the interphase sector between “cognitive” and “visceral/emotional” portions of the cingulate cortex, specifically on the left side, in depressed patients who had not responded to cognitive behavior therapy (CBT) or venlafaxine treatment.
Other evidence points to a preeminent role of right cortico-subcortical functioning in the modulation of mood and in response to antidepressant treatment. Kennedy et al.
63 described changes in brain glucose metabolism associated with 16-week-long treatment either with venlafaxine or CBT. Right subgenual/ventromedial frontal increase in metabolism was observed in patients responding to CBT, whereas decreases in metabolism in the right posterior subgenual cingulate were associated with response to venlafaxine. Notably, reduction in metabolic activity bilaterally in the orbitofrontal cortex was associated with response to either treatment modality. In contrast, decreased metabolism restricted to the left orbitofrontal cortex was observed irrespective of treatment group or treatment outcome (i.e., left hemispheric changes were irrelevant to the response of depressive symptoms). The Kennedy et al.
63 study extends asymmetrical brain metabolic findings to the right thalamus, a subcortical structure that is a component of the circuit involved in mood regulation. These findings, as well as the fact that Mr. A responded to unilateral right stimulation of Brodmann’s area 25, are also congruent with the observation that the right dorsomedial PFC is activated relatively selectively in a wide range of emotional tasks, including recollection of affectively meaningful life events,
64 attention to subjective feelings,
65 and processing of emotion-related meanings.
66 In this fMRI study, healthy subjects were presented a series of pictures combined with relevant captions resulting in positive, negative, and neutral affective connotations. Presentation of negative picture-caption pairs was associated with activation of a series of cortical and subcortical structures, in all cases localized to the right hemisphere: medial and right middle frontal gyri (Brodmann’s area 9), right anterior cingulate gyrus (areas 24 and 32), and right thalamus.
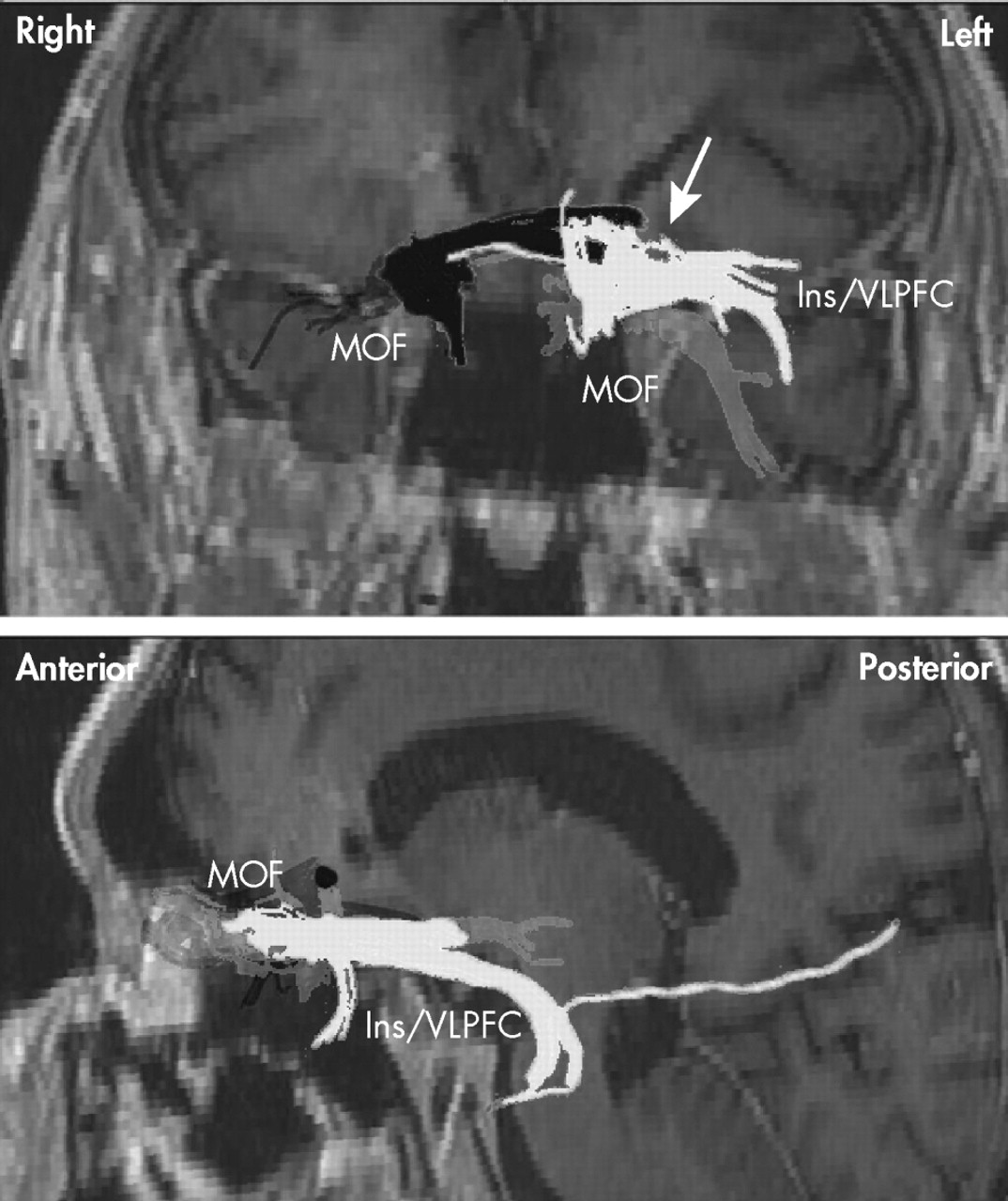
66Our provisional observation of prolonged depression remission with right, but not left, unilateral stimulation of the subgenual cingulate cortex (Brodmann’s area 25) is concordant with those findings in response to antidepressants and the induction of negative affect in healthy individuals, and suggests that efficacy of DBS in Mr. A was entirely explained by its effects on the right cerebral hemisphere. Some of the implicated right-sided structures are in the vicinity of the midline, so the efficacy of right DBS might also be explained, at least in part, by bilateral effects of a single electrode placed on that side. This is best appreciated by observing the trajectory of tracts in the vicinity of—and thus presumably affected by—the DBS electrodes in Mr. A (
Figure 3 ). As seen in this figure, neuronal tracts affected by each electrode show ipsilateral projections to the PFC. Tracts neighboring the right electrode, however, seem to additionally project contralaterally to a greater extent than those in the vicinity of the left electrode in this patient. This emphasizes the need to further define the tracts critical for mood regulation
67 and perhaps suggests that, in the future, attempts at regulating brain circuits might need to be “customized” to match the interindividual variability in anatomical projections. This approach has already been proposed for subthalamic nuclei DBS in idiopathic Parkinson’s disease.
68 Regardless of the reasons for the present observation of asymmetric contribution of right versus left DBS to the amelioration of depressive symptoms, Mr. A’s case probably points to a need to take into account hemispheric asymmetries in models of depressive symptom generation.
If further studies confirm, that just as is the case with attention,
48 the right hemisphere serves a preeminent role in normal mood regulation and clinical depression, the question arises whether lateralization applies to all components of Mayberg’s model of depression delineated above
5,
25 or if instead “interoceptive” (subcortical/paleocortical) or “exteroceptive” (neocortical) nodes are preferentially lateralized in their contribution to mood regulation.
Asymmetrical Central Representation of Visceral Function and Its Relationship With Regulation of Mood and Emotion
There is recent evidence favoring lateralization in the control of abnormal visceral sensation in the context of mood dysregulation. Coen et al.
69 described the patterns of brain activation during the processing of painful and nonpainful visceral stimuli (esophageal distention) in different affective states. Evidence for a dominant role of the right hemisphere in the bodily correlates of negative emotion was obtained that specifically involves the right anterior cingulate (Brodmann’s areas 24 and 32), right anterior insula, and inferior frontal gyrus.
69 Gut sensations evoking increased heart rate, low-frequency/high-frequency heart rate variability ratio, and plasma epinephrine, an autonomic pattern characteristically associated with depression,
70 –
73 are associated with increased activation of right insula, right orbitofrontal cortex, and right parahippocampal gyrus, as well as subcortical and brainstem structures.
74 This further suggests a right hemispheric dominance in the control of visceral sensation associated with negative mood and the autonomic profiles characteristic of it.
Induction of negative mood has been associated with hyperactivity of right insula and prefrontal cortical structures in healthy volunteers as well.
4 Consistent with this view, Giesecke et al.
75 observed that symptoms of depression correlate with pain-evoked activation of the right anterior insula. Craig
76,
77 summarized evidence explaining the lateralization of emotion processing and suggested that the right insular cortex might be the principal CNS structure responsible for sentience and self-consciousness. He proposed that prosencephalic emotional asymmetry in turn reflects an asymmetrical representation of homeostatic activity, ultimately explained by asymmetries in the autonomic nervous system hierarchy including peripheral sympathetic and parasympathetic structures. Vagal afferents innervate the nucleus of the solitary tract, whereas sympathetic afferents terminate in lamina I of the dorsal horn of the spinal cord. From there, axons innervate the basal (parasympathetic) and posterior (sympathetic) portions of the ventromedial nucleus of the thalamus. This topographical organization is present in primates, and is extremely well-developed only in humans.
76,
78 –
80 Ultimately, the left anterior insula is activated by afferent information related to parasympathetic functions, whereas the right anterior insula is activated by afferent information associated with sympathetic activity (e.g., pain).
76 Thus, activity in the right prosencephalon is associated with energy expenditure, sympathetic activity, arousal, aversive behavior, and survival emotions, whereas activity in the left forebrain is associated with energy nourishment, parasympathetic activity, relaxation, appetitive behavior, and affiliative emotions.
77 There is, in fact, a growing body of evidence that suggests that left and right forebrain structures are associated with positive and negative affect, respectively.
81 It has been suggested that this, in turn, reflects asymmetries in autonomic afferent input onto limbic structures.
82,
83 The concept behind the correlation between anatomical segregation of afferent autonomic input and cerebral involvement in the processing of emotions is the cornerstone of the James-Lange theory, which maintains that emotional feeling states are initiated by autonomic afferent information reaching consciousness.
84,
85 This theory has received support recently because of evidence that peripheral autonomic clues are crucial for motivated behavior.
86,
87Complete suppression of otherwise treatment-refractory depressive symptoms in Mr. A by means of right DBS (presumably blocking neural transmission in forebrain limbic structures on that side
88 ) is consistent with this growing body of evidence on lateralization of emotional regulation and also with preliminary observations that another neuromodulation tool useful in treatment-resistant depression, vagus nerve stimulation, might exert its beneficial effect via activation of left forebrain structures and reciprocal inhibition of right forebrain structures.
39,
76 Although we do not have functional brain imaging studies available to demonstrate the effects of DBS on either side of the brain in Mr. A, we have collected data on cardiac autonomic activity in resting conditions (
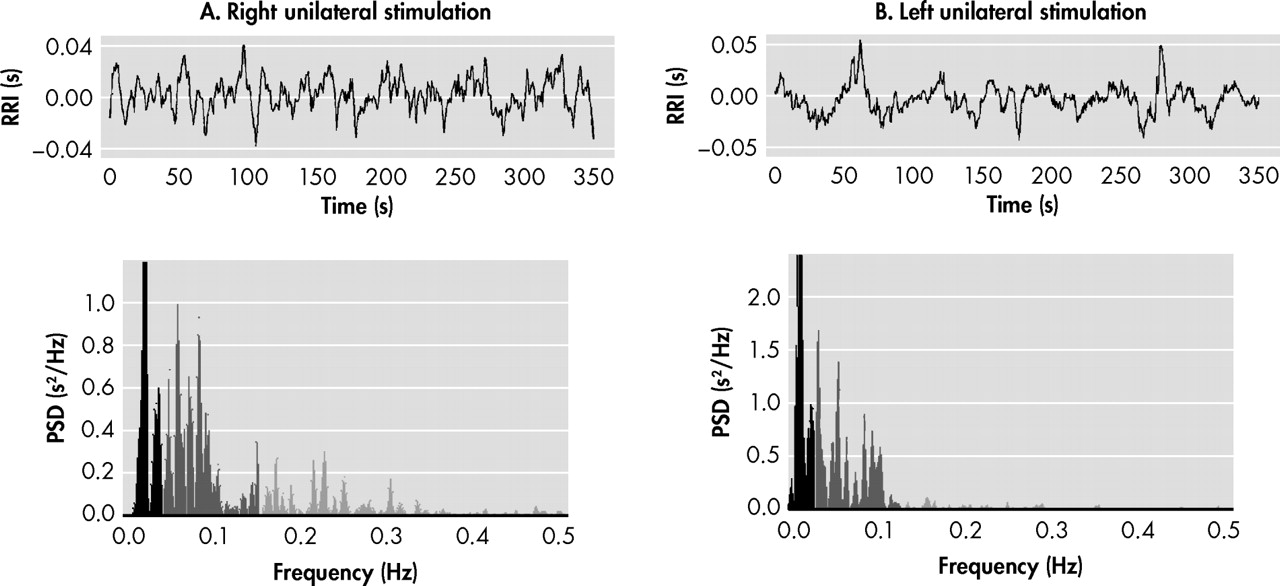
Figure 4 ). Using analysis of heart rate variability,
89 we identified high-frequency (parasympathetic) and low-frequency (baroreflex, but predominantly sympathetic) influences on the sinus node (Biomedical Signal Analysis Group, Department of Applied Physics, University of Kuopio, Finland). As shown in
Figure 4, unilateral right DBS was characterized by remarkably lower low-frequency/high-frequency ratio (an estimation of the sympathovagal balance on the heart)
89,
90 than unilateral left DBS. As expected, heart rate was higher in the latter condition, further suggesting increased sympathetic and/or decreased vagal input to the heart. If confirmed, these observations suggest that emotional well-being depends not only on “top-down” but also “right-left” modulation of prosencephalic activity, and would permit reconceptualization of older studies on the effect of brain lesions in the production of depressive symptoms. The common observation of left lesions being associated with the development of depression might, in light of this model, be explained less by the interruption of brain circuits on the left side than by unopposed influences of right forebrain and limbic structures, physiologically associated with negative affect, sympathetic activity, and energy expenditure. Stimulation of the unencumbered right hemisphere, resulting in inhibition of local and remote structures within these circuits,
1 may help to restore interhemispheric balance.
Summary and Recommendations for Future Research
Although still relatively small in number, there is burgeoning evidence of the efficacy of DBS in the treatment of the most severely treatment-refractory depressed patients.
10,
41 The current case adds to this database, and like previous reports it demonstrates the potential utility of this intervention in those patients who have failed to respond to past adequate treatment with psychotherapy, a variety of antidepressants, and ECT. Although the present observation needs to be confirmed in double-blind studies, as Mr. A’s psychiatrist was not blind to changes in stimulation parameters, this case raises important questions about laterality of mood regulation and unilateral versus bilateral DBS treatment, which warrant further study. The present case example and the bulk of evidence highlight the preeminence of cortical and subcortical structures in the right hemisphere in the generation and maintenance of depression. This hypothesis remains to be confirmed with studies specifically addressing this question, including animal models of the disease as appropriate.
Acknowledgments
Presented in part at the Annual Congress of the World Psychiatric Association in Florence, Italy, April 1–4, 2009. Dr. Mayberg is consultant to Advanced Neuromodulation Systems/St. Jude Medical, and intellectual property holder and licensor in the field of deep brain stimulation. In the past year, Dr. Nemeroff has served on the Boards of Directors of Novadel Pharma, Mt. Cook Pharma, the George West Mental Health Foundation, and the American Foundation for Suicide Prevention, on the Scientific Advisory Boards for AstraZeneca, CeNeRx, PharmaNeuroboost and NARSAD, and holds equity in Novadel Pharma, Corcept, CeNeRx, Reevax and PharmaNeuroboost. He holds two patents, one on transdermal delivery of lithium (US 6,375,990B1) and one on ex vivo monoamine transporter measurement (provisional filing). Dr. Guinjoan’s work was supported in part by a National Agency of Promotion grant (PICT-2007-01643). The other authors report no competing interests.