H untington’s disease is an autosomal dominant, neurodegenerative disorder resulting from an expanded trinucleotide cytosine-adenine-guanine (CAG) repeat (≥36 glutamines), coding for the mutant protein huntingtin on chromosome 4p16.3.
1 Symptomatic treatment is widely available although no cure is possible. Clinical features of Huntington’s disease consist of movement, neuropsychiatric, and cognitive disorders. Disease progression causes a decline of daily functioning, and patients ultimately become totally dependent on the help of others.
Apathy is a common neuropsychiatric feature of Huntington’s disease.
2 –
4 Reported prevalence of apathy in Huntington’s disease varies from 34% to 76%, depending on disease stages examined and assessment methods used,
5 and its prevalence and severity increase with disease progression.
6 Apathy has been described both as a symptom (i.e., of mood disorder, altered level of consciousness, or cognitive impairment) and as a syndrome.
7,
8 An apathy syndrome is defined as a disorder of motivation, with loss of or diminished goal-directed behavior, cognitive activity, and/or emotion, as well as functional impairments that are attributable to the apathy.
9,
10 Clinically, apathy has been related to decline in activities of daily living causing a great burden of disease and distress in caregivers,
11 also after adjusting for the presence of motor and cognitive deficits.
12,
13In the present study, we aimed to assess the prevalence of apathy in Huntington’s disease mutation carriers and comparison noncarriers. Furthermore, we investigated sociodemographic, clinical and neuropsychiatric correlates of apathy comparing Huntington’s disease mutation carriers with apathy to those without apathy.
METHODS
Participants
Between May 2004 and August 2006, Huntington’s disease mutation carriers were recruited from the outpatient departments of Neurology and Clinical Genetics of the Leiden University Medical Center and from a regional nursing home. Subjects with a CAG repeat length of 36 or more repeats were considered positive for Huntington’s disease mutation carriership.
The design of the study has been described in detail elsewhere.
14 In short, of 361 known subjects, 45 outpatients were untraceable, 17 subjects were excluded or were deceased, and 89 refused to participate because of various reasons. Fifty-six subjects appeared to be noncarriers. After the assessment, two more subjects were excluded because of a missing motor score. Thus, 152 Huntington’s disease mutation carriers and 56 noncarriers were included in the present analysis. All subjects gave written informed consent. The study was approved by the Medical Ethical Committee of the Leiden University Medical Center.
Instruments
Assessment of Apathy
Apathy was assessed using the semistructured Apathy Scale (
Figure 1 ).
15 The Apathy Scale is a modified version of the Apathy Evaluation Scale
7 and consists of 14 questions read by the interviewer, measuring different features of apathy in the 2 weeks prior to the interview. As patients with apathy often lack insight into their behavior, we also used caregivers’ information. The subject and his or her informant are provided with four possible answers: not at all, slightly, some, and a lot. The total score of the Apathy Scale ranges from 0–42 points, with higher scores indicating greater apathy. The Apathy Scale has shown good interrater reliability and good test-retest reliability, as well as high internal consistency in patients with Parkinson’s disease.
15 We used an Apathy Scale total score ≥14 points to characterize subjects as apathetic, and those scoring below this cutoff score as nonapathetic.
15,
16Sociodemographic and Clinical Characteristics
Information on sociodemographic and clinical characteristics of mutation carriers and comparison subjects was collected in a standardized manner. Global functioning was assessed with the Total Functional Capacity scale of the Unified Huntington’s Disease Rating Scale (UHDRS).
17 The Total Functional Capacity scale consists of five questions assessing employment; capacity to handle financial affairs, to manage domestic chores, to perform activities of daily living; and the care level provided (range=0–13 points; lower scores indicate poorer functional abilities).
18Assessment of Motor Function
Neurological examination was done by a neurologist with experience in Huntington’s disease, blind for the genetic status of the subject and according to the motor section of the UHDRS.
17 The UHDRS-motor consists of 15 items and several subitems that are rated on a scale from 0 (normal) to 4 (severe) points. The total UHDRS-motor score is the sum of all individual motor ratings (total score range=0–124 points; higher scores indicating worse motor performance).
The confidence level of the UHDRS-motor was used to define subjects as premotor symptomatic (confidence level score =0 or 1 point) or motor symptomatic (confidence level score =2–4 points).
Assessment of Depression
Because symptoms of apathy may overlap with depression, we assessed the presence of depression (major depressive disorder and dysthymia) according to the criteria of the DSM-IV.
19 Psychiatric assessment was done by a psychiatrist (EvD) or a trained research assistant under his supervision. Raters for psychiatric and cognitive function were informed about the genetic status of the subjects, because nondisclosure could considerably influence subjects’ answering questions about symptoms that are directly related to mutation carriership.
The Dutch translation of the computerized version of Composite International Diagnostic Interview (CIDI, Version 2.1) was used to classify depression according to DSM-IV criteria.
20 The CIDI was not administered in subjects with a score <18 points on the Mini-Mental State Examination (MMSE), since the CIDI cannot be reliably administered to patients with such a severe cognitive dysfunction. In these subjects the presence of a depression was assessed clinically, based on the psychiatric examination, medical reports, and information of caregivers.
Neuropsychological Assessment
The MMSE, Symbol Digit Modalities Test (SDMT), Verbal Fluency Test, and Stroop Color and Word Tests were administered to assess cognitive function.
The MMSE consists of 11 items and has been found to be reliable and valid in assessing global cognitive function. Scoring range of the MMSE is 0–30 points with lower scores indicating worse global cognitive performance.
21The SDMT examines attention, working memory, and visuo-verbal substitution speed.
22 Subjects have 90 seconds to write down the number that matches each of the geometric figures, which are printed on several lines.
The Verbal Fluency Test is sensitive to frontal executive dysfunction and subtle degrees of semantic memory impairment.
23 Subjects are instructed to generate as many words as possible in 1 minute. A total Verbal Fluency Test score of less than 30 words is considered abnormal.
The Stroop Color and Word Test was used to measure a person’s sustained attention in three conditions: color naming, word reading, and naming the color of the ink of an incongruous color name (interference).
24 For each condition the subject had 45 seconds, and the total of all right answers was scored, with maximum 100 points per condition.
Statistical Analyses
Data are presented as n (%), mean (± SD) or median (interquartile range [IQR], i.e., 25th to 75th percentiles) when appropriate. Chi-square tests for categorical data, t tests for independent samples with normal distributions, or nonparametric Mann-Whitney U tests were conducted to compare mutation carriers and noncarriers. Mutation carriers with and without apathy were compared to determine correlates of apathy using univariate logistic regression analyses. Odds ratios (OR) and their corresponding 95% confidence interval (CI) were computed. Total Functional Capacity, UHDRS-motor, MMSE, SDMT, Verbal Fluency Test, and Stroop Color and Word Test scores were divided into two groups using a median split. A p value of <0.05 was considered statistically significant.
Because of a strong collinearity between the Symbol Digit Modalities Test, Verbal Fluency Test, and Stroop Color and Word Test, a new variable for executive cognitive function was computed by averaging the 4 index z scores (i.e., subtracting the mean from an individual raw score and then dividing the difference by the standard deviation).
Multiple logistic regression analysis, identified by a forward stepwise selection procedure, was used to determine the independent correlates of apathy. For this analysis, the following variables with p value <0.05 in the univariate regression analysis were used: sex, age, Total Functional Capacity score, UHDRS-motor score, use of antidepressants, use of neuroleptics, use of benzodiazepines, presence of depression, MMSE score, and executive cognitive function score. The overall use of psychotropic medication was not entered, because of the inclusion of the three medication subcategories. Statistical analysis was carried out by means of the SPSS for Windows, release 16.0.
RESULTS
Sociodemographic and Clinical Characteristics
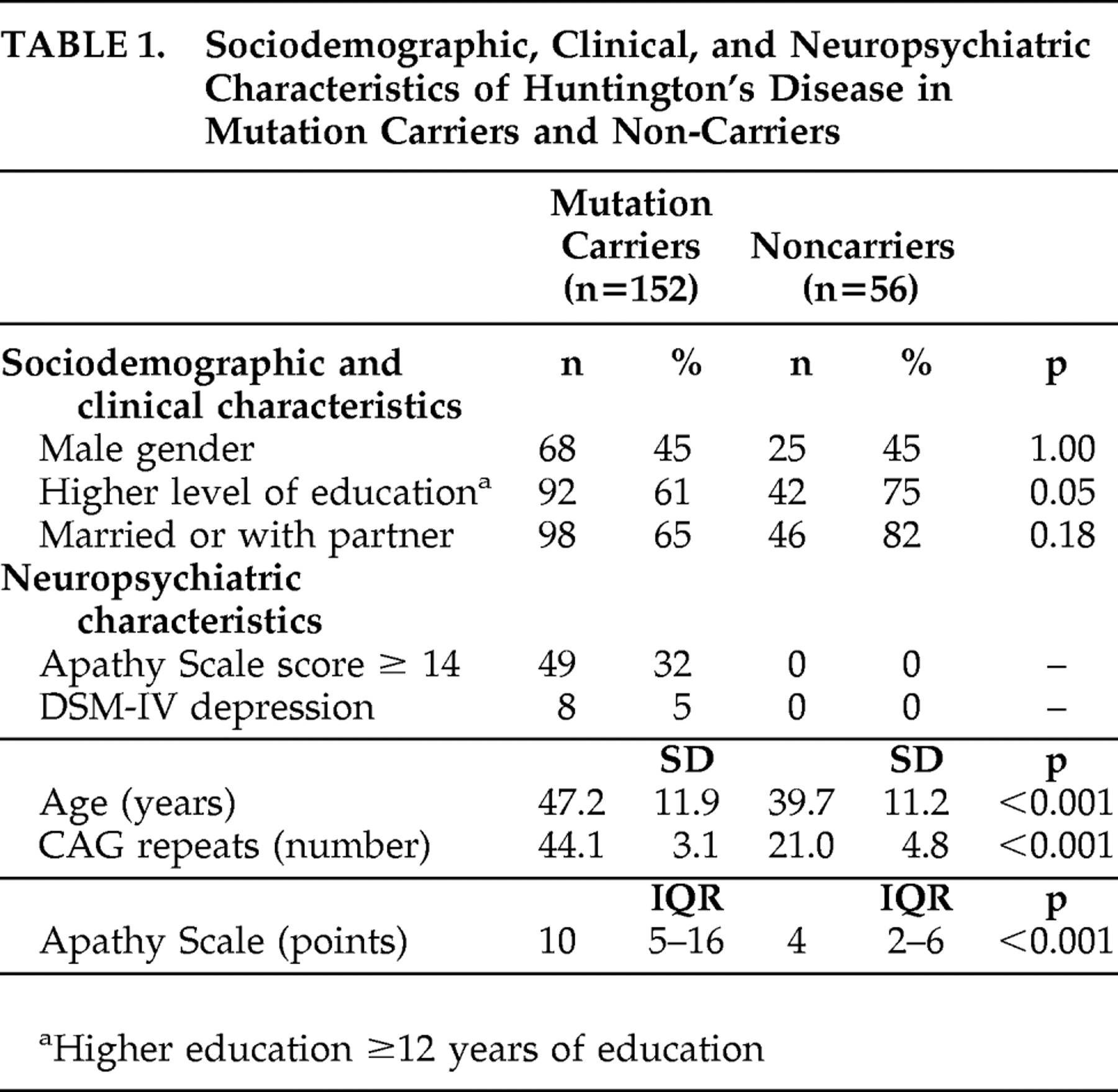
The sociodemographic, clinical, and neuropsychiatric characteristics of 152 Huntington’s disease mutation carriers and 56 noncarriers are shown in
Table 1 . Mutation carriers were older and had significantly more symptoms of apathy than noncarriers (
Table 1 ). Mutation carriers also had more often a formal DSM-IV diagnosis of depression compared to noncarriers. Assessment of the CIDI was not possible in 12 mutation carriers because of severe cognitive impairment (MMSE score <18 points). Using information of caregivers, medical reports and clinical impression during the assessment, two of these 12 mutation carriers were diagnosed as depressed.
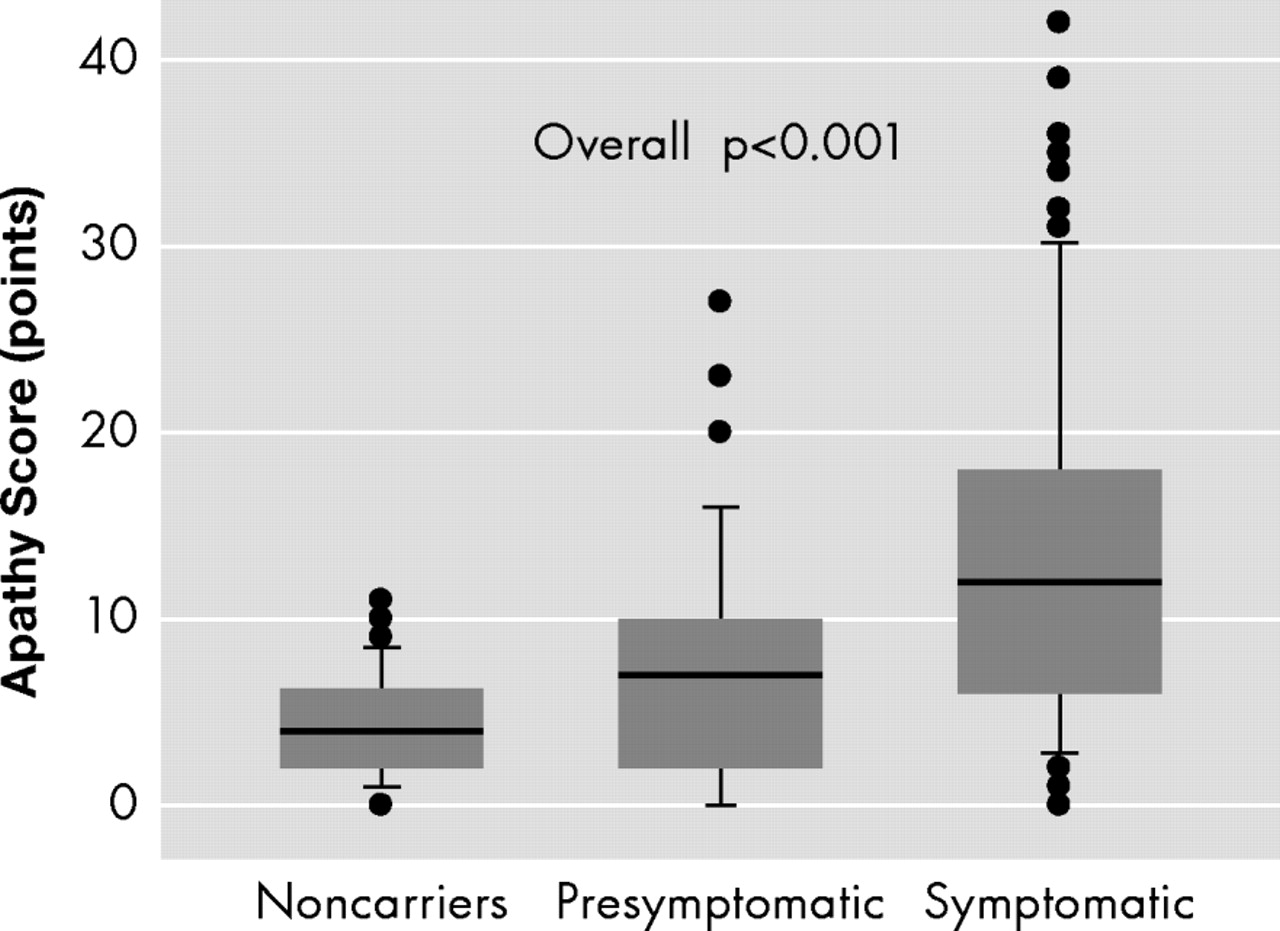
Mutation carriers with motor symptoms showed significantly more symptoms of apathy than premotor symptomatic mutation carriers and noncarriers, and premotor symptomatic mutation carriers showed significantly more symptoms of apathy than noncarriers (overall p<0.001) (
Figure 2 ).
Huntington’s Disease Mutation Carriers With and Without Apathy
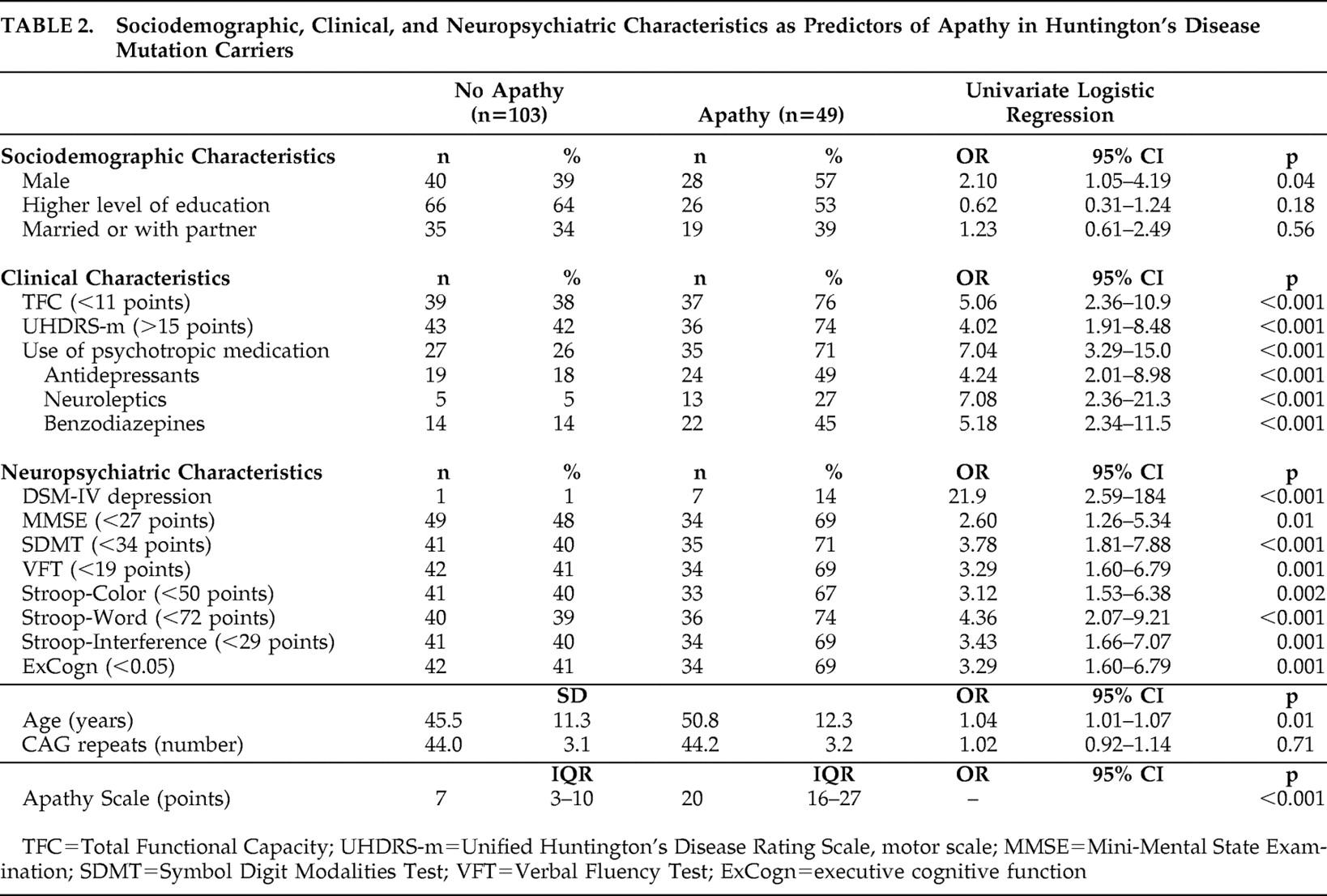
Forty-nine mutation carriers (32%) were considered apathetic (median Apathy Scale score =20 points; IQR=16–27), whereas 103 mutation carriers (68%) were not (median Apathy Scale score =7 points; IQR=3–10) (
Table 2 ).
Univariate regression analysis showed that, in comparison with nonapathetic mutation carriers, apathetic subjects were more often male and older, had a lower Total Functional Capacity score, had a higher UHDRS-motor total score, used more psychotropic medication, were diagnosed more often as depressed, and showed worse global and executive cognitive function.
Independent Correlates of Apathy in Huntington’s Disease Mutation Carriers
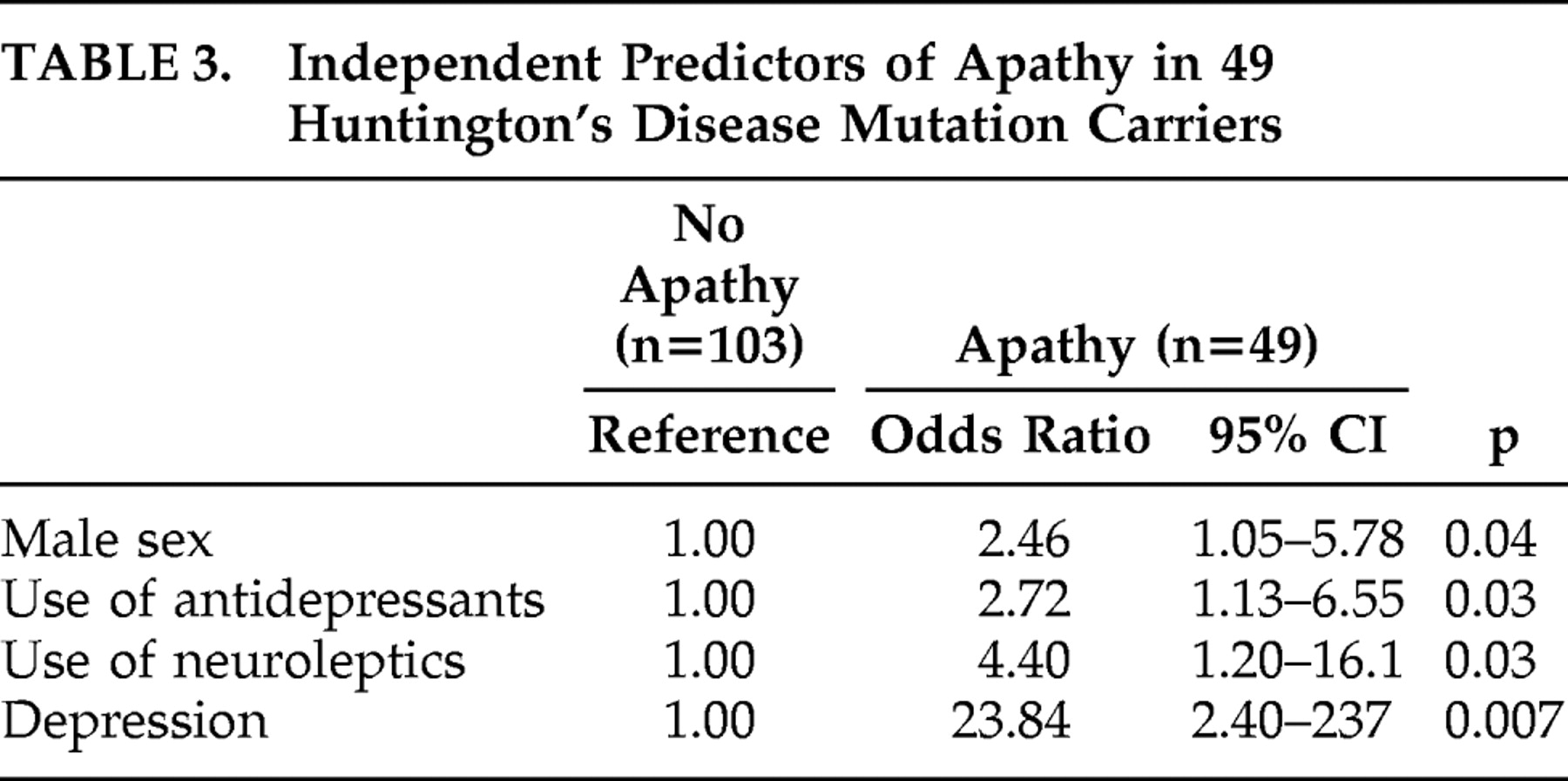
Using logistic regression analysis, male sex, higher use of both antidepressants and neuroleptics, and the presence of depression were statistically significant independent correlates of apathy in a multivariable analysis (
Table 3 ).
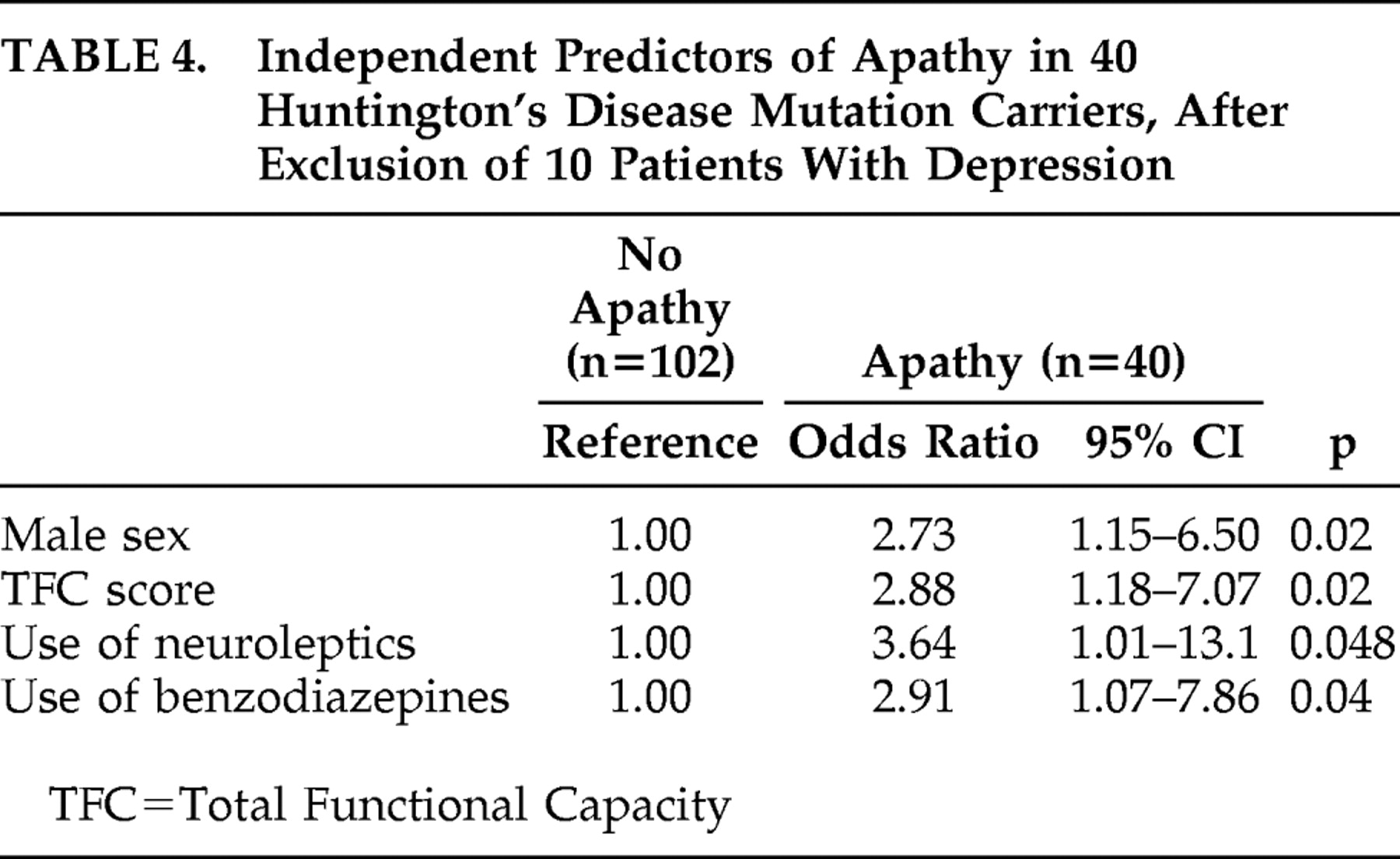
In addition, a sensitivity analysis was conducted to evaluate the robustness of our model and to eliminate the possibility of confounding influences of depression on the correlates of apathy. As described above, eight subjects had a formal diagnosis of depression according to the CIDI (seven subjects in the apathetic group and one subject in the nonapathetic group), and two without the CIDI assessment were clinically depressed (both in the apathetic group). After exclusion of these 10 subjects with depression, higher use of antidepressants was no longer independently associated with the presence of apathy. However, male sex and higher use of neuroleptics were still independent predictors of apathy, together with lower Total Functional Capacity score and higher use of benzodiazepines (
Table 4 ).
DISCUSSION
The results of our study confirm that apathy frequently occurs in Huntington’s disease with a prevalence of 32% in mutation carriers compared to 0% in noncarriers. Mutation carriers with apathy were more likely to be male, of older age, and using more psychotropic medication. When comparing mutation carriers with apathy to those without apathy, significantly more depression, worse total functioning with more severe motor and cognitive symptoms, and increased use of psychotropic medication were shown. After exclusion of mutation carriers with depression, the independent associations with the presence of apathy in Huntington’s disease mutation carriers were male sex, worse global functioning, higher use of neuroleptics, and higher use of benzodiazepines.
Apathy and Depression
The relationship between apathy and depression varies across diagnostic groups and depends on assessment tools used.
25 Apathy can be a clinical sign of depression but can also occur independently. In Huntington’s disease, apathy has been shown to be associated with the presence of depressed mood,
3 but inconsistently.
11,
26,
27 Contrary to our findings, one other study using the CIDI found no association between a formal diagnosis of depression and apathy in patients with traumatic brain injury.
28 In another study applying a factor analysis of the Montgomery and Åsberg Depression Rating Scale
29 in patients with acquired brain damage, “negative symptoms” of depression were highly associated with apathy, whereas “depressed mood” or “somatic symptoms” were not.
30Apathy and the Use of Psychotropic Medication
The presence of apathy was associated with higher use of different types of psychotropic medication. The association with the use of antidepressants—not surprisingly—disappeared after the exclusion of subjects with depression. Higher use of neuroleptics remained independently predictive, together with higher use of benzodiazepines.
Since this study has a cross-sectional design, we cannot conclude whether the use of psychotropic medication is a cause or consequence of apathy. In clinical practice, antidepressants may be prescribed as a treatment for apathy, but in our study their use seems to be related to presence of depression. Development of apathy as a side effect of the use of neuroleptics and benzodiazepines is very well possible, due to their blunting and sedative effects, which may result in lethargy and fatigue.
Furthermore, distinguishing apathy from depression is of clinical importance because of potential differences in the use of pharmacological and nonpharmacological interventions. Pharmacotherapy for depression may improve the clinical profile but can also have a counteractive effect on apathy.
31 For example, serotonin reuptake inhibitors may increase apathy and withdrawal from engagement with the environment.
32To date, no specific treatments for apathy are known. Preliminary studies suggest that apathy may respond to pharmacotherapy with stimulants, dopamine agonists, acetylcholinesterase inhibitors, or NMDA-receptor antagonists.
33,
34Apathy and Cognitive Function
Using univariate analysis we found an association between presence of apathy and worse cognitive function. This result is in line with a previous study among patients with early Huntington’s disease that found severe deficits in attention, executive function, and episodic memory to be related to apathy.
35 In other neurodegenerative disorders, an association between apathy and cognitive dysfunction has also been described. For example, apathy correlated with initiation-perseveration in subjects with progressive supranuclear palsy,
36 and a correlation between apathy and worse performance on several cognitive tests measuring executive cognitive function in Parkinson’s disease has been reported.
27 Also, in Alzheimer’s disease, patients with apathy performed worse on the SDMT and the Stroop-Interference test than those without apathy.
37In patients with dementia and apathy, a faster cognitive and functional decline has been found compared to patients without apathy.
34 In an earlier study,
6 we found significantly more apathy in advanced stage Huntington’s disease. Therefore, apathy may be a sign of disease progression in Huntington’s disease, including progressive motor and cognitive impairments, and worse global functioning, but longitudinal studies are needed to investigate precise relationships.
The strengths of this study are a relatively large study population with Huntington’s disease, the use of a comparison group, and the use of specific and validated measurement tools in a standardized interview. However, there are some limitations that warrant discussion. First, this study involved the analysis of cross-sectional data which precludes conclusions about the direction of causality. Second, as discussed before, assessment of the Apathy Scale was done during a clinical interview with the mutation carrier and an informant, whereas the CIDI was assessed in absence of the informant. This may have reduced the validity of the CIDI assessment, as Huntington’s disease patients may have a lack of insight into their own behavior and feelings. Another limitation was that some of the explanatory variables were rather strongly intercorrelated and that the automated variable selection method in the logistic regression may therefore have produced models of somewhat limited stability. Further, all subjects volunteered to participate in this study, which may have led to an underestimation of the prevalence of apathy in Huntington’s disease patients due to selection bias, as subjects who did not respond to the invitation to participate in the study may have been more apathetic.
We conclude that apathy is highly prevalent in Huntington’s disease and is strongly associated with the presence of depression, worse global functioning, and the use of psychotropic medication (especially neuroleptics and benzodiazepines). Therefore, we advise to evaluate the use of all psychotropic medications to exclude an iatrogenic cause of apathy.