Elderly patients scheduled for a surgery because of urological reasons are often characterized by multimorbidity, changed neurotransmitter balance, and/or polypharmacy. Additionally, medications with anticholinergic properties (e.g., sedatives, narcotics, and antibiotics) are regularly applied in perioperative conditions and may lead to an increased anticholinergic burden.
2 The concept of an anticholinergic burden addresses a situation in which a variety of medications with anticholinergic actions are prescribed, leading to anticholinergic toxicity which can be detected as increased serum anticholinergic activity (SAA) by a muscarinic anticholinergic radioreceptor assay,
3 independently of the origin of anticholinergic burden.
Using this assay technique, previous work has suggested a link between SAA and cognitive decline. Thus, several studies reported a correlation of SAA with cognitive impairment during depression
4 or dementia
1,
5 as well as with delirium in intensive care patients.
6,
7 Recently, particular interest has been directed to the anticholinergic burden in surgical patients due to its possible relationship with an increased risk of transient and persistent postoperative cognitive dysfunction.
2,
6 Cerebral cholinergic deficit is considered one of the main reasons for abrupt cognitive decline and postoperative dysfunctions including delirium.
8,
9 Postoperative cognitive impairment is of particular significance as it is associated with worsened outcome in hospitalized patients, including prolonged hospital stay and increased mortality.
10,
11 Because SAA reflects the cumulative binding capacity of muscarinic receptors to acetylcholinergic substances,
3 most of these studies support the hypothesis that increased SAA is related to an anticholinergic burden caused by anticholinergic medications.
12 –
14However, the question of the relationship between endogenous and exogenous factors modifying SAA levels is controversially discussed due to the finding of elevated SAA in patients taking no known anticholinergic medications.
15 Therefore, this work raised the possibility that in addition to medications, an endogenous source of anticholinergic activity may exist. Pre- and postsurgical stress could be such a factor. Stress is mainly mediated through high blood cortisol levels. In parallel with neurotransmitters including acetylcholine, it is well known that steroid hormones influence different functions of the nervous system.
16 Whether elevated cortisol or cholinergic deficits are causally, or as an epiphenomenon, related to cognitive impairment is not clear. However, it is shown that sustained high glucocorticoid levels may be associated with the progression of CNS diseases such as stroke, depression, and neurodegenerative diseases.
17 Furthermore, a growing body of evidence showed that the basal tonus of the hypothalamic-pituitary-adrenal (HPA) axis also increases with aging, promoting hypercortisolemia, which is of interest since aging is recognized as a risk factor for cognitive disorders.
18 Although it is well known that, in addition to acetylcholine, glucocorticoids play an important role in learning and memory functions,
17 –
19 the role of cortisol during transient delirium is not clear. Likewise, the interaction between acetylcholinergic neurotransmission and glucocorticoid actions in relation to cognitive processes is still unknown, in particular under the conditions of perioperative surgical stress.
Thus, the central question of the present study is to investigate whether acute stress expressed by increased blood cortisol is associated with patients’ acetylcholinergic levels and cognition in perioperative surgical settings for urological reasons.
METHODS
Patients
We recruited patients between April and December 2007 at the Department of Anesthesiology at University of Heidelberg in Germany after obtaining written informed consent. This study was approved by the local ethics committee and institutional review board and was performed in accordance with the ethical standards of the Declaration of Helsinki.
To exclude the effect of sexual hormones on glucocorticoids we included only male patients. Patients older than 18 admitted for elective surgery in urology were included with the exception of patients with psychiatric or neurological disorders, such as dementia, depression, or schizophrenia, and stroke patients or those with a history of alcohol abuse. Furthermore, we excluded patients with pronounced hearing and/or visual impairment problems and non-German-speaking people because the neuropsychological tests were in German. In all, five patients were not included in the present study because of the exclusion criteria. Therefore, in accordance to power analysis (see statistics), we included 30 patients in the present study.
Characterization of the Patients
For preoperative evaluation a structural study tool was employed that included the patients’ age, sex, body weight and height, American Society of Anesthesiologists classification, and hospital admission diagnosis. Lengths of hospital stay and hospital mortality were also recorded. In addition, preoperative and postoperative medication as the number of drugs with potential and known anticholinergic properties according to previous studies
19,
20 was recorded. We included the number of preoperative anticholinergic medications that had been administered continuously for longer than 4 weeks before patients’ inclusion in our present study (long-term effect of medication).
Blood and CSF Sampling and Spinal Anesthesia
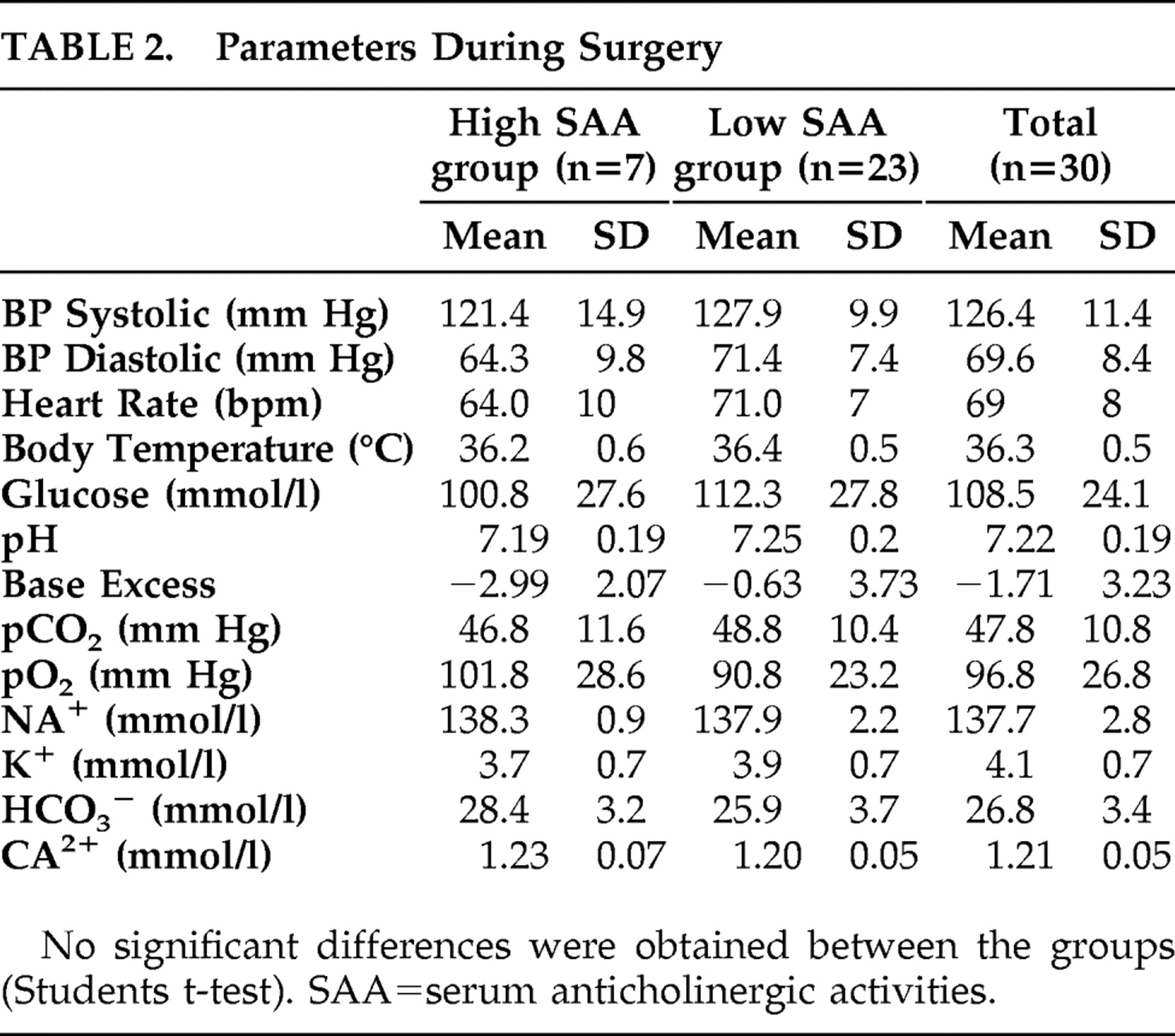
Blood samples were taken three times: (I) 1 day before surgery, (II) shortly before spinal anesthesia, and (III) on the first postoperative day between 9 and 11 a.m. Additionally, patients’ physiological (blood pressure, heart rate, and body temperature) and arterial blood parameters (pO 2, pCO 2, glucose, pH, base excess, standard bicarbonate, potassium, calcium, sodium, hematocrit, and hemoglobin levels) were determined at the end of surgery (IV).
CSF samples were taken once between 8 a.m. and 10 a.m., shortly before spinal anesthesia (respectively to II) strictly by the same investigator (PT) in a standardized manner. All patients were premedicated orally with 7.5 mg midazolam at 24 hours and 1 hour before the planned surgery. Two milliliters of venous blood and 1 ml of CSF were taken under local anesthesia (subcutaneous: mepivaciane 1%) and stored at 4°C for a maximum of 1 hour before further processing.
Scoring Tests
The investigator involved in neuropsychological testing (JM) was trained by a psychologist in scoring procedures; cross-center observation and cross-scoring of test protocols were used to assure data quality before the beginning of the study.
Delirium was assessed in every patient by the same investigator using the German translation of the Abbreviated Mental Test, the confusion assessment method, and the Delirium Index
21 –
23 on the basis of ICD-10 and DSM-IV criteria by evaluating four items: acute onset of cognitive changes with a fluctuating course, inattention, disorganized thinking, and altered level of consciousness.
Neuropsychological testing included the Mini-Mental State Examination (MMSE).
24 Working memory capacities were determined using the Auditory Verbal Learning Test (word list: immediate and delayed) and the Digit Span Task (forward and backward) from the German Nuernberger Alters-Inventar.
25 The duration of the MMSE and time for performing the whole battery of tests was recorded.
The testing was performed 1 day before surgery and on the first postoperative day immediately before blood was taken for biochemical analysis.
Blood Analysis
The venous blood samples (2 ml) were centrifuged at 7,000 rpm for 10 minutes. Thereafter, the supernatant was taken and stored together with vials containing the CSF for a maximum of 1 month at −80°C until anticholinergic activities were determined.
An investigator blinded to all clinical data assessed the plasma anticholinergic activities by competitive radioreceptor binding assay as described by Tune and Coyle.
3For measurement of total cortisol levels, 100 μL of plasma or CSF was extracted with 1 ml of diethyl ether. The organic phase was evaporated in a stream of nitrogen. The residue was analyzed applying a commercial cortisol enzyme immunoassay kit, which operates on the basis of competition between an HRP-cortisol conjugate and the cortisol in the sample (Oxford Biomedical Research, Rochester Hills, Michigan). Intra-assay and inter-assay coefficients of variation were 7.1% and 3.4%, respectively.
Statistical Analysis
Power analysis, assuming a clinically important difference of 15% in cortisol levels between the groups, suggested that 30 patients were required for the study (α=0.05; 1−β=0.8). Comparisons of normally distributed data were made using the unpaired two-tailed t test (subgroup comparison). The data for descriptive variables were analyzed by chi-square test; the data from blood analyses were compared for statistical significance using ANOVA followed by the Tukey’s post hoc test. Bivariate correlation analysis was done according to Pearson.
Statistical analyses were performed on SPSS, version 16.0 (SPSS Inc., Chicago). The results are expressed in
Table 1 and
Table 2 . A p value of <0.05 was considered to be statistically significant.
DISCUSSION
Significant correlations between peripheral (serum) and central (CSF) compartments for cortisol and anticholinergic activities are one precondition that both peripheral measured markers are adequately reflecting the CNS situation.
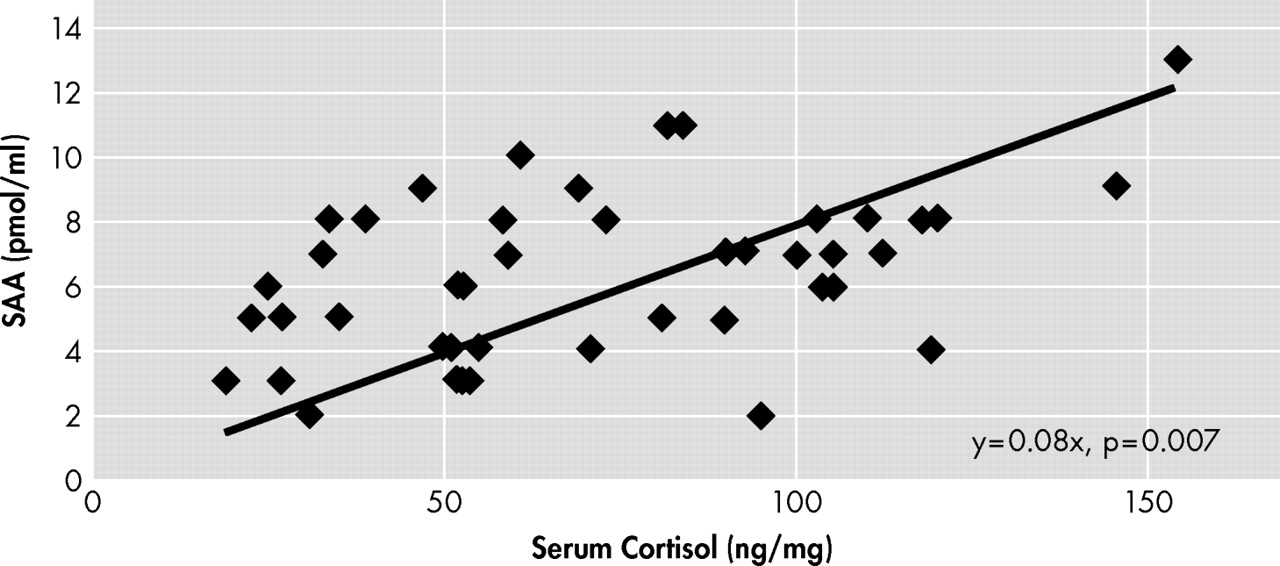
We show for the first time in our knowledge that there is a linear positive correlation between cortisol levels and SAA in patients scheduled for surgery for urological reasons. Furthermore, we have shown that the number of preoperative anticholinergic drugs in patients’ history is also clearly associated with higher SAA levels. This result is consistent with most other studies
12 –
14 and supports the hypothesis that increased SAA is related to the anticholinergic burden caused by anticholinergic medications. In addition, the results confirm our findings of a previous study with a smaller sample that the relationship between serum and CSF anticholinergic activities helps validate SAA.
26As stated above, however, endogenous sources of SAA related to fever, acute infection, or stress have been postulated independently of anticholinergic medication.
15,
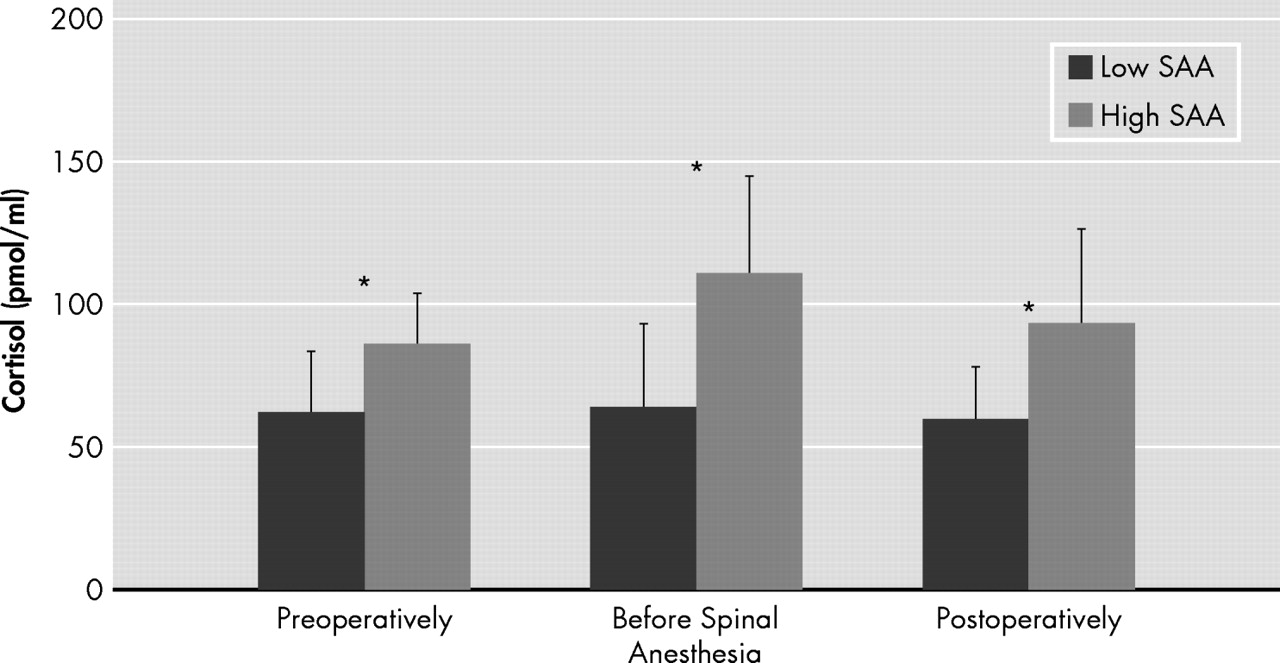
27 We did not find a correlation between SAA and patients’ medical history as defined by the American Society of Anesthesiologists (ASA) guidelines. However, only patients with ASA classification I to III were included in this investigation. From endogenous factors, serum cortisol levels seem to play an important role for interpretation of SAA because high serum cortisol concentrations were associated with increased SAA levels (
Figure 4 ). Thus, the present data also support the hypothesis that some individuals may have anticholinergic activity in the absence of anticholinergic drugs.
15,
28Taking both results together, our data confirm the hypothesis that both endogenous as well as exogenous factors had an influence on patients’ SAA levels. Thus, sources of the anticholinergic burden can be highly individual, and therefore the SAA may be considered as a conglomerate of anticholinergic properties of exogenous and endogenous origin. However, the generalizability of our findings needs to be estimated carefully. Our study was done only with urological patients. Further investigations on other patient populations should be conducted to justify our conclusion in relation to the interpretation of the role of SAA in general.
In a chronic state, the deleterious role of high serum cortisol levels leading to cognitive impairment is shown.
16 –
18 Chronic stress, which can have a physiological and a psychological source, causes a permanent activation of the sympathetic nervous system, which in turn activates the HPA axis and causes elevation in serum cortisol. The role of acute stress in the development of postoperative delirium and cognitive dysfunction, however, is controversially discussed.
29 –
32 Elevated serum cortisol levels have been associated with delirium, for instance, in Cushing’s disease and high-dose steroid treatment.
29 Only a few small studies have examined the association between serum cortisol levels and delirium in general medical and surgical settings, and the results are controversial.
30,
31 One further report suggests that individuals who fail to suppress in the dexamethasone suppression test appear to be at increased risk for delirium.
32 Thus, the lack of suppression in the dexamethasone suppression test is a sign of an overactive HPA axis. Therefore, the results of the study seem to be fully consistent with our present findings. In line with other biomarkers, however, careful control of patient characteristics should be evaluated, and further studies will be required to confirm the importance of hypercortisolism as a biomarker for delirium.
If stress, as mediated by elevated cortisol, may be an endogenous source of anticholinergic activity, an interrelation between glucocorticoids and cholinergic system is assumed. Therefore, we used this SAA assay according to Tune and Coyle
3 to detect a direct effect of cortisol in a preliminary study. Our results (data not shown) showed that exogenous applied cortisol had no direct effect on muscarinic receptors under in vitro conditions. Therefore, another—likely an indirect—interaction between cortisol and cholinergic system seems likely. Little is known about the interaction of glucocorticoids and acetylcholine. In the periphery, glucocorticoids may interfere with the storage and inactivation of acetylcholine (e.g., cholinesterase activity may be up-regulated by glucocorticoids).
33 Furthermore, long-term treatment with glucocorticoids increases synthesis and stability of junctional acetylcholine receptors on innervated cultured human muscle.
34 The human acetylcholine esterase gene was demonstrated to include a glucocorticoid-responsive element. Furthermore, evidence exists that alternative splicing forms of the acetylcholine esterase may be associated with stressful events.
35Diverging results have been reported on the question of whether SAA correlates with cognitive dysfunction. Although most of the studies suggest that there is a clear association between SAA levels and cognitive impairment,
4 –
7 we could not confirm this effect in our present study; despite some high SAA levels in our included patients, no postoperative cognitive dysfunctions were detected. To investigate the role of SAA in relation to cognition, other groups of patients undergoing surgery under general anesthesia or with a longer in-hospital stays should be investigated with a more detailed battery of neuropsychological tests. Furthermore, a regression analysis is now planned to perform a risk factor analysis in undergoing studies. Additionally, the severity of illness in our middle-aged patients was moderate and the duration of the surgical procedures was relatively short (<1 hour). Thus, the risk of postoperative cognitive dysfunction in the presented study design was low. However, our previous studies on critically ill intensive care patients could also not detect an association between high SAA levels and delirium in postoperative delirium.
36 Therefore, the association between SAA and patients’ cognitive function still remains controversial and should be further investigated in larger patient populations.
It can be concluded from the present study that sources of the anticholinergic burden seem to be individual, and SAA therefore may be considered as a conglomerate of anticholinergic properties of endogenous and exogenous origin. The results of the present study pointed out that for interpretation of diagnostic benefit of SAA, different factors should be taken into account as we have shown for anticholinergic medication and cortisol.
Further studies are necessary for investigation of the role of SAA as a diagnostic marker for surgical patients with a high stress level or a high anticholinergic burden caused by polypharmacy with anticholinergic medication.