The development of investigative techniques and imaging methods has determined the pace of the scientific study of the neural correlates of consciousness.
13 Back in 1929, Berger first demonstrated the presence of spontaneous electrical activity by use of a crude electroencephalogram (EEG).
20 Visualization of anatomy has progressed through computerized tomography (CT) and low-resolution MRI to 7-tesla MRI. Of particular importance is the use of functional imaging that allows the measurement of neuronal activity: functional MRI (fMRI), positron emission tomography (PET), and single-photon emission tomography (SPECT). They are based on the principle that brain metabolism is characterized by coupling of cerebral blood flow and oxygen-extraction/consumption.
21 This can be measured using radioactive fluorine-labeled glucose, as in the case of FDG-PET, or hemoglobin-oxygenation (BOLD [blood oxygen level-dependent] signal), as in the case of fMRI.
22Thalamo-Cortical Connections
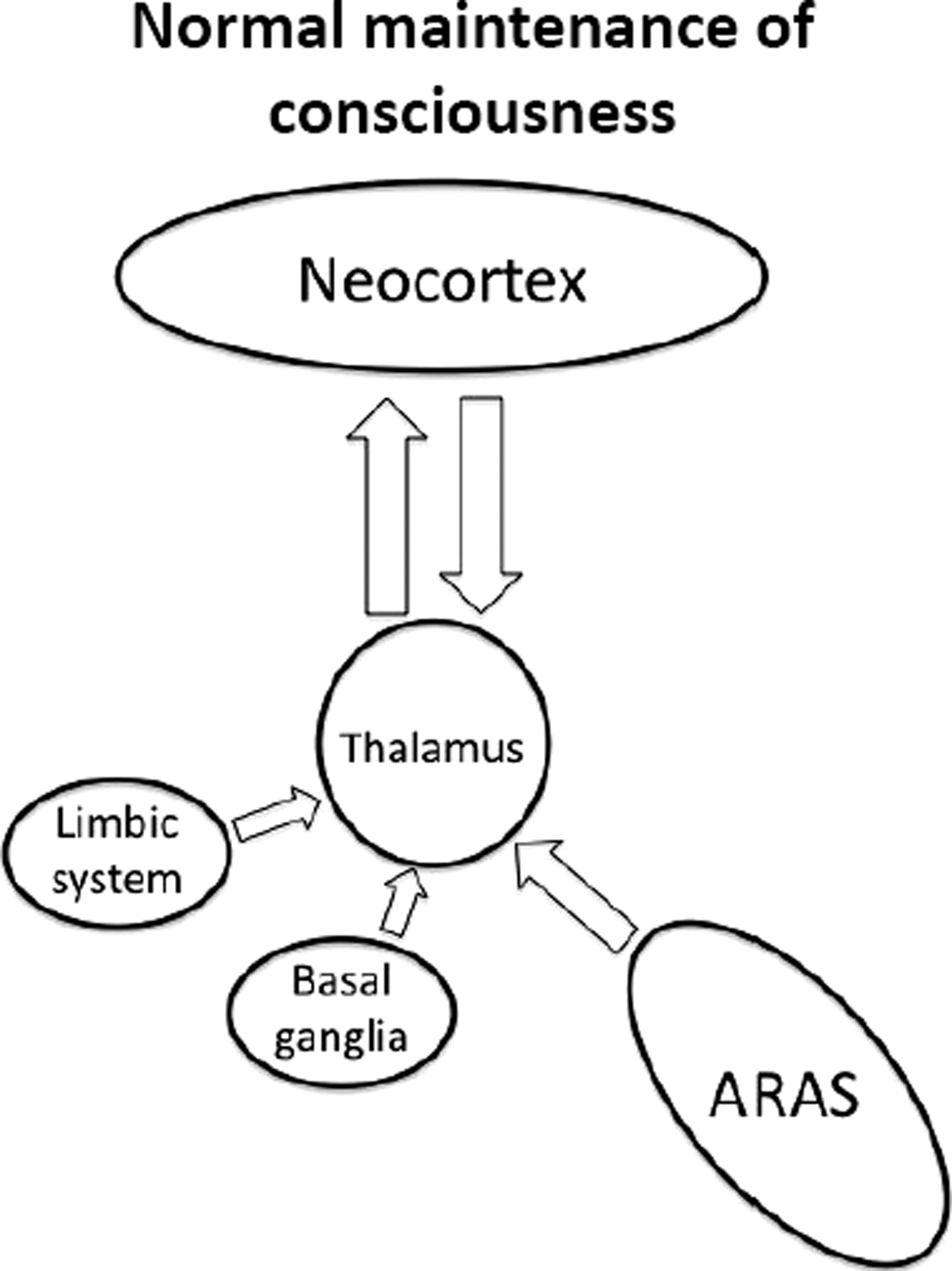
There are multiple projections from the thalamus that are important for cortical activity.
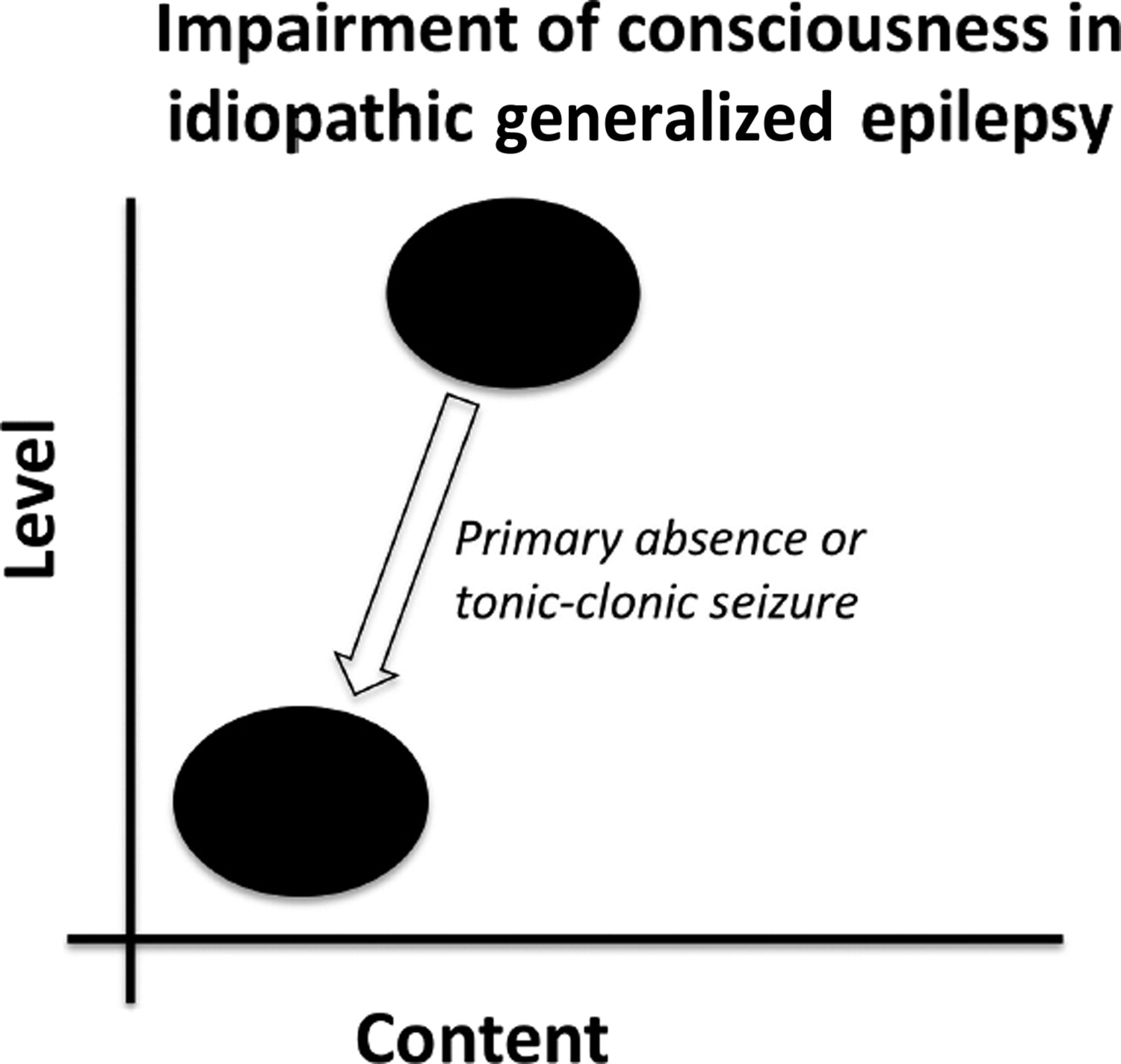
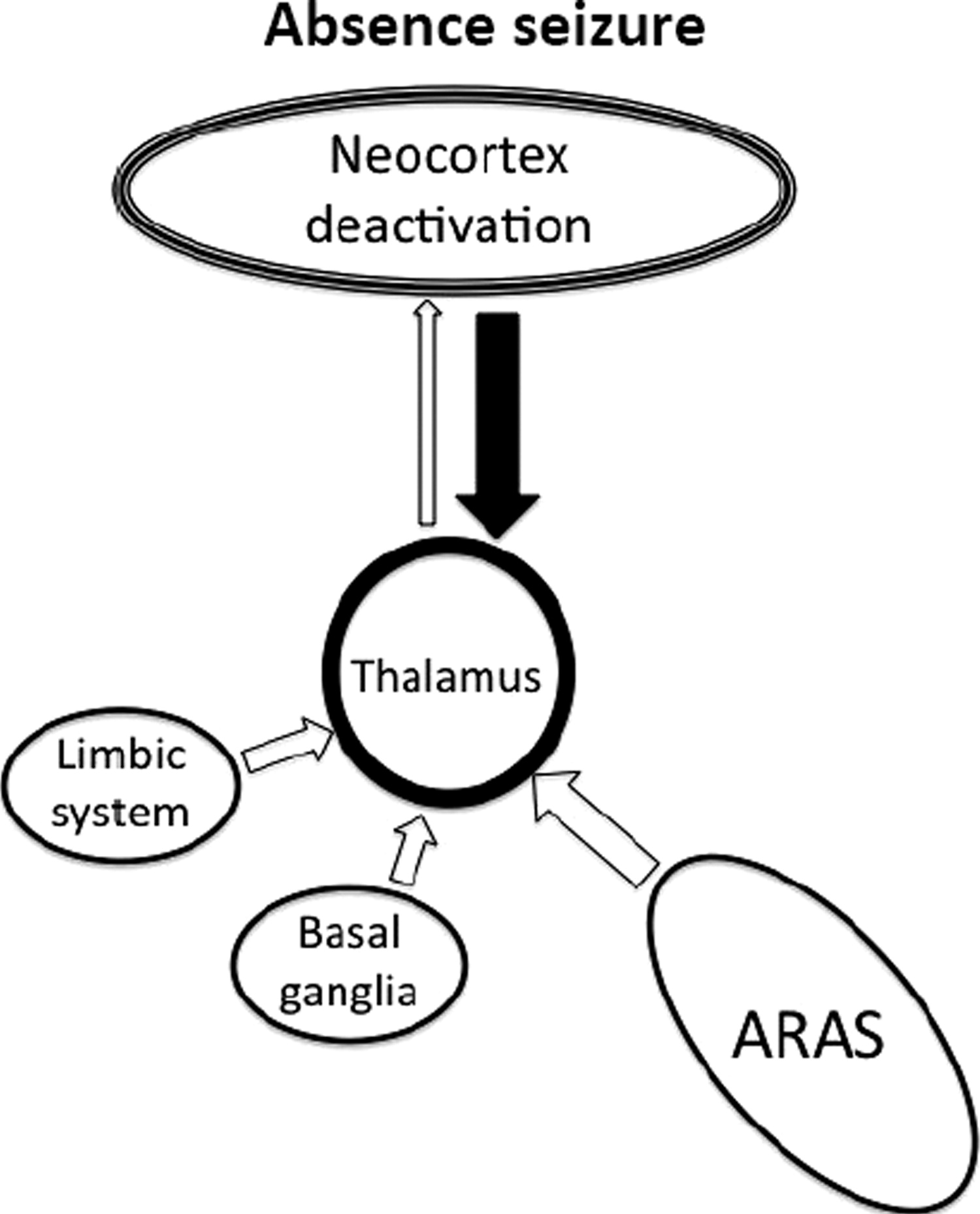
17,33 Absence seizures are characterized by a widespread 3- to 4-Hz “spike and wave” pattern on EEG that is thought to result from abnormal thalamo-cortical activity (
Figure 4).
34 This can be elicited by stimulation of the intralaminar thalamic nuclei,
35,36 and most (although not all) absence seizures result in impairment of consciousness.
13 It can therefore be inferred that regulated activity in these parts of the thalamus is required for maintaining consciousness.
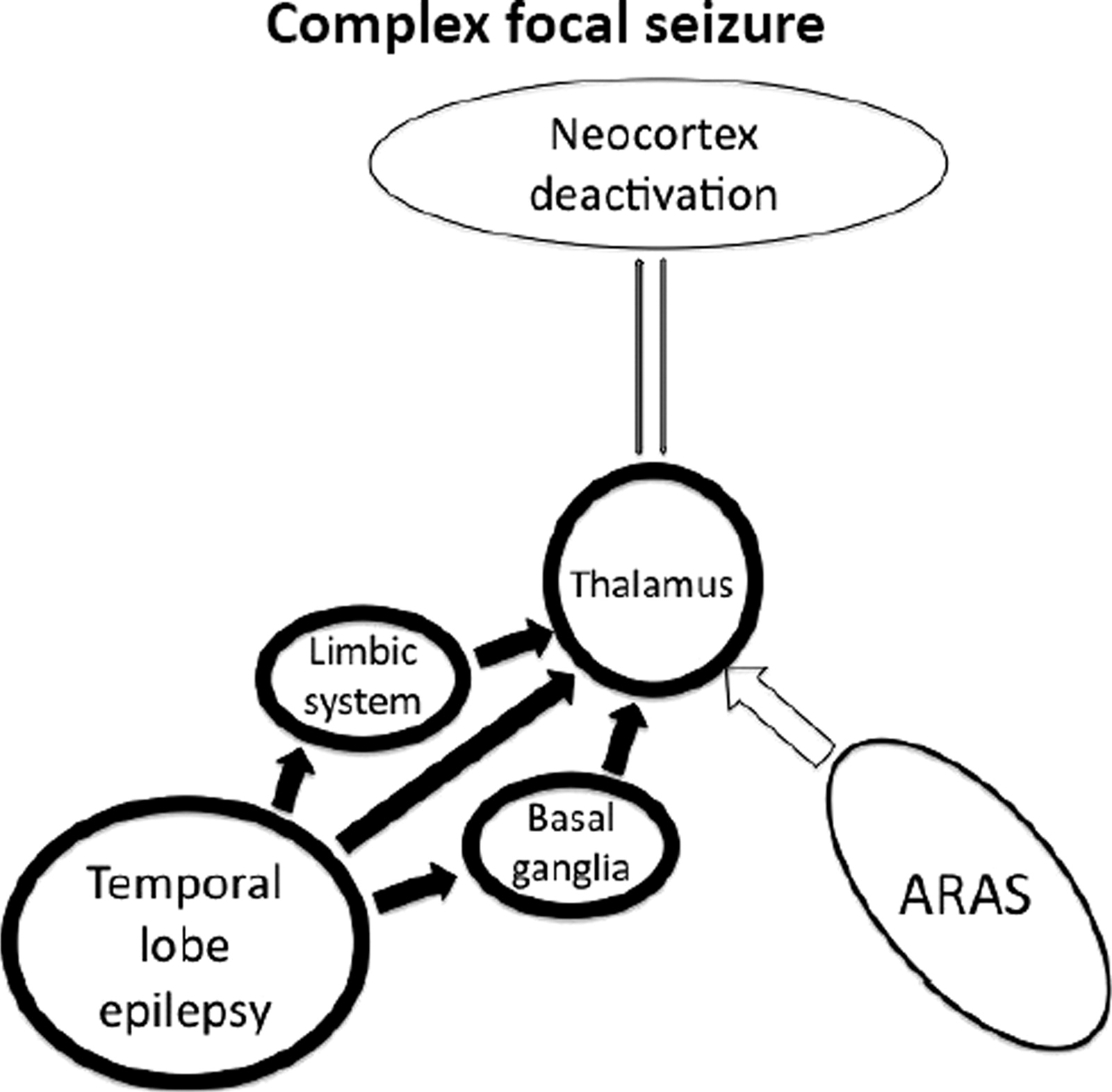
Spread to the thalamus is thought to be partly responsible for loss of consciousness during complex focal seizures of temporal lobe origin. There are established neuronal connections between the mesial temporal lobe and midline subcortical structures.
18,37,38 Likewise, a functional correlation has been found between reduced consciousness level during temporal lobe seizures and hyperperfusion of the thalamus.
15 Taken together, these findings support the theory that propagation from the temporal lobe is responsible for the impaired level of consciousness.
Cerebral Cortex and the Baseline State
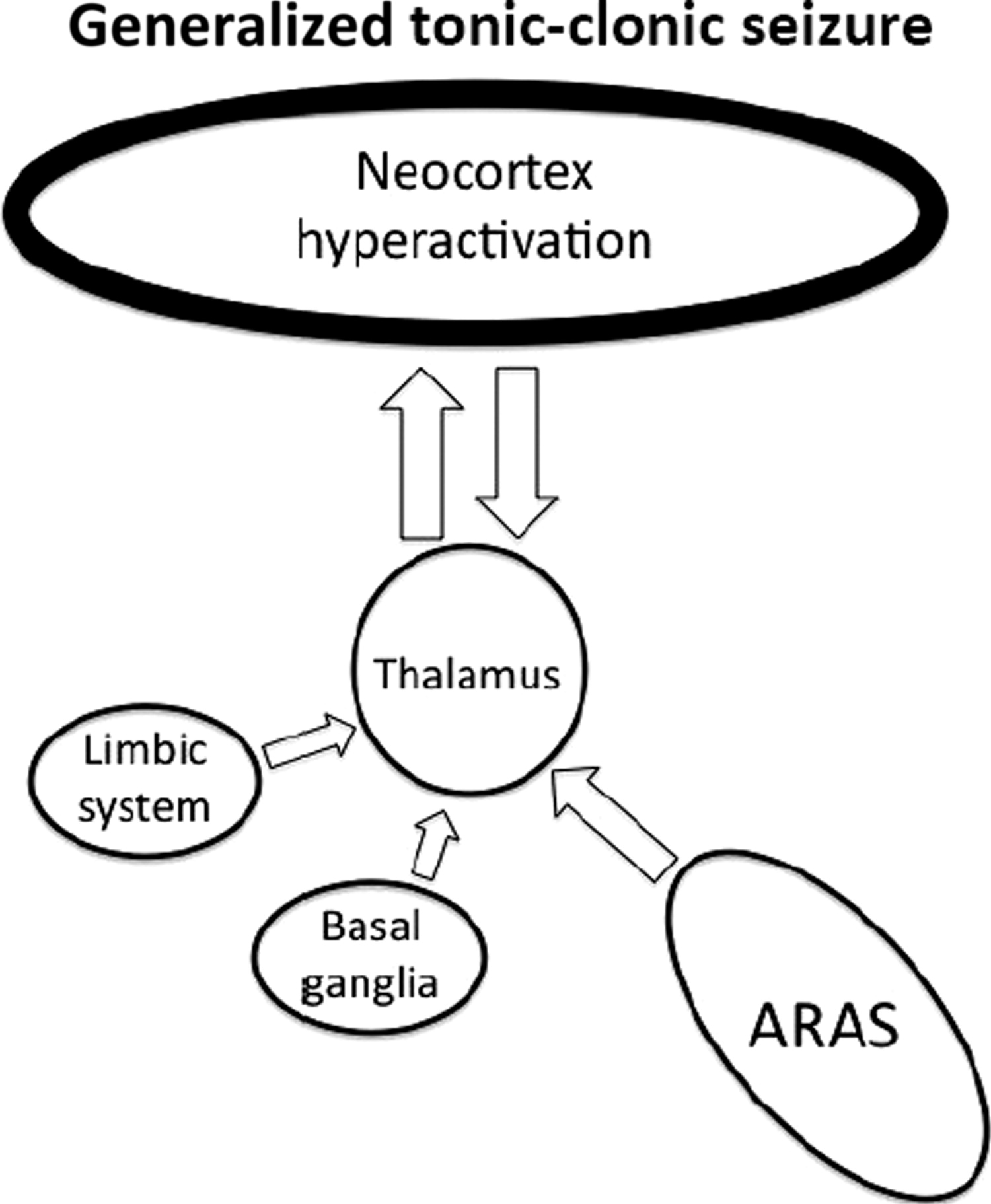
The cerebral cortex is known as the location for higher cognitive processing; thus, it is not surprising that it is also central to consciousness. In generalized seizures, large areas of the cortex are diffusely involved, and consciousness is almost always impaired.
39 However, others have noted generalized seizures with localized areas affected,
40,41 and there are reports of patients who have suffered GTCS without loss of consciousness.
42 Therefore, not all cortical regions appear to be equally involved in consciousness.
Although it has long been known that there is hemispheric dominance for many functions (e.g., speech), it has been traditionally difficult to elucidate consistent data on dominance for consciousness. Studies of patients who have undergone hemispherectomy show that only one hemisphere is needed for consciousness.
43 Using amytal hemispheric inactivation, some researchers have suggested that the left side is dominant for consciousness.
44 A more recent study, using video-electroencephalography (video-EEG), showed that consciousness is more frequently preserved if the right temporal lobe is affected, rather than the left.
45 However, most of these studies used verbal response to assess consciousness, which is known to be biased toward the left side in the majority of patients.
7 Of note, in one study, no hemispheric dominance was observed when noxious stimuli were used,
46 and other work demonstrated bilateral cortical involvement to be necessary for impaired consciousness.
47Functional neuroimaging studies have consistently found that the cortical areas that are selectively deactivated during seizures are the fronto-parietal association areas, including the medial prefrontal cortex, the posterior cingulate cortex, and the precuneus.
48–50 These regions are normally concerned with planning and association of visuospatial imagery with episodic memory.
51,52 Raichle and colleagues
53 noted that previous investigators had found these same areas to have a reduced cerebral oxygen-extraction fraction (i.e., they show a relative deactivation) when subjects shift from a baseline control state to a cognitively active state where they perform a non–self-directed task. This idea was developed further to suggest that the fronto-parietal association areas form part of the brain's “default state,” such that they are selectively activated in the absence of other activity. The same areas are known to be affected in diseases with impaired sensory awareness (e.g., Alzheimer's disease
54) and are required for peripheral awareness. However, it is yet to be established whether these cortical regions are required for self-awareness, being meta-reflection, an integral part of consciousness.
Studies using SPECT imaging of GTCS have demonstrated that increased activity in the fronto-parietal association cortices correlates with loss of consciousness
14 (
Figure 5). This is in contrast with absence seizures, which are known to have reduced cortical activity in these regions.
13 These findings support the importance of the medial prefrontal cortex, posterior cingulate cortex, and precuneus in maintaining the level of awareness, by showing that their abnormal activation or deactivation can impair the normal function of consciousness.
Norden and Blumenfeld
55 have summarized the evidence on the brain mechanisms responsible for the alterations of consciousness during complex focal seizures of temporal lobe origin as the “network inhibition hypothesis.” The propagation of temporal lobe seizures to the thalamus interrupts the normal ARAS function, which, in turn, results in reduced activity of the fronto-parietal association cortices (
Figure 6).
This idea is supported by the finding of slow-wave EEG activity in these cortical areas during complex focal seizures of the temporal lobe.
56 Although the exact mechanism behind this phenomenon is unknown, modulation of inhibitory or excitatory inputs seems to be involved.
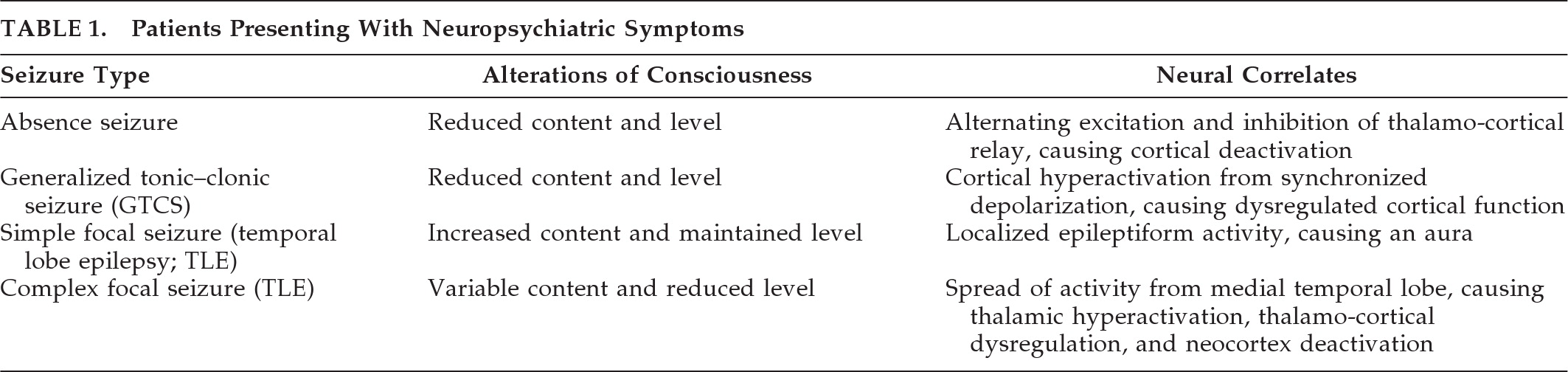
12 The neural correlates of the alterations in level and content of consciousness in the most common seizure types affecting consciousness are summarized in
Table 1.
Implications for Clinical Practice
As definitions of consciousness are fine-tuned and our understanding of epilepsy widens, classification of epilepsy is modified.
57 Is “generalized” an appropriate name for seizures that are known to have selective cortical effects? Furthermore, within the current setting of translational research, we should aim to use our understanding of the neurobiological mechanisms underlying consciousness to modulate the impact of epilepsy on patients. Pharmacological manipulation of neuronal function has been the mainstay of epilepsy management for many years. Drugs could thus be targeted to act on the regions specific to loss of consciousness, to block the pathways responsible for epileptiform activity propagation, or to influence receptors that have a key role in sustaining consciousness. This may permit maintenance of consciousness, even if seizure frequency could not be reduced.
A more invasive method of preserving consciousness in patients with epilepsy may be via use of deep brain stimulation (DBS). On the basis of previous experience in movement disorders, some research groups have begun to develop DBS approaches for epilepsy, vegetative state, and traumatic brain injury, with an aim of improving consciousness functions.
58–60 Further work on this technique may ultimately improve management of seizures that affect consciousness in patients with refractory epilepsy.
The “Explanatory Gap” and Neurophilosophy
Our understanding of consciousness and its impairment has come a long way since the time when epilepsy was considered a manifestation of witchcraft.
61 We have developed a sophisticated conceptual framework to address the “mind–body problem” within the realm of neuroscience. We have identified some of the key brain regions that are involved in generation and maintenance of conscious experience.
62 However, modern neuroscience is still facing a conundrum: we know that specific cerebral activation-states correlate with the experience of subjective conscious contents, but the crucial link between the two is missing: this is known as the “explanatory gap.”
63 Physicists have proposed quantum theories as a solution, where the movement of dissociated electrons (the physical domain) generates consciousness (the non-physical domain).
64–66 Philosophers have analyzed evidence from psychophysiological experiments (e.g., Libet's famous demonstration of the neural time factor
67) to conclude that perhaps the gap is not what we believe it to be because our perception of consciousness has been misled.
68 In the 1970s, Benjamin Libet and his group conducted a series of investigations into human consciousness; one of these experiments demonstrated that the unconscious electrical processes in the brain, called “readiness potential” (
Bereitschaftspotential), actually precedes conscious decisions to perform volitional acts, which are retrospectively felt to be consciously motivated by the subject. These experiments pose challenging questions to the neuroscientific quest for a comprehensive explanation of the brain mechanisms underpinning consciousness. Neuroscientific investigations in both experimental models and pathophysiological states in humans must aim to fill in the blanks between our current knowledge and what other disciplines tell us about conscious experience.
Neurophilosophy is the term used to describe the interplay between neuroscience and philosophy. Philosophy can help to inform neuroscientists and propose avenues for exploration, whereas advances in neuroscience can change the way that philosophers think about certain concepts.
69 For instance, neurosciences approach consciousness within the conceptual framework of reductionist philosophy: that is, changes in conscious states will ultimately be explained via cellular and molecular mechanisms.
70 It can be argued that reductionism leads to a deterministic theory of human behavior, where all our actions are determined by molecular events. This view, which is in line with the results of Libet's experiments, clearly poses a challenge to the philosophical concept of “free will.” Moreover, if consciousness is reduced to the activity of neurons, then it does not transcend the natural realm of physics and, by definition, fails to cross the boundary between metaphysical and physical: the main argument against classical dualism.
11 The neurophilosophy of consciousness has important implications for future scientific inquiry in this area: according to the reductionist model, the continued development of electrophysiological and functional neuroimaging paradigms addressing brain activity in patients with (epilepsy-induced) alterations of consciousness will be one of the most promising ways forward in the consciousness-research arena.