The prevalence of anxiety syndromes in persons with Parkinson's disease (PD) is markedly higher than in healthy and comparably disabled elderly control subjects; lifetime prevalence estimates are as high as 50%.
1,2 Specific anxiety disorder subtypes appear to contribute to this pattern.
2–5 For instance, Nuti et al.
1 diagnosed panic disorder in 30% of PD subjects, as compared with only 5.5% of non-PD control subjects. By contrast, in the same sample, there were no group differences in the prevalence of generalized anxiety disorder or obsessive-compulsive disorder.
An increase in select anxiety disorder subtypes may be associated with discrete pathological processes in PD or a shared vulnerability to both anxiety and PD. Thus, anxiety disorder subtypes could serve as phenotypic markers for investigating the pathophysiology of PD and its genetic underpinnings. In a general-practitioner's practice, patients who developed PD were more likely to present with anxiety, autonomic symptoms, and sleep disturbance, during a prodromal period of 4–6 years before the onset of overt motor symptoms.
6 This complements findings from epidemiologic studies showing an association between anxiety disorders and PD; in two large studies, anxiety disturbances earlier in life were associated with a significantly higher relative risk of developing PD.
7,8 Finally, a recent study demonstrated an increased risk of depressive and anxiety disorders in relatives of patients with PD.
9 These studies did not always apply Diagnostic and Statistical Manual for Mental Disorders, 4th Edition (DSM-IV) criteria for anxiety disorders. Therefore, it is unclear whether the familial risk is attributable to specific anxiety disorder subtypes.
Although long considered to be a sporadic and nongenetic disease, recent genome-wide association studies have identified several genetic risk factors associated with PD.
10,11 Familial aggregation studies show an increased risk of PD among first-degree relatives of patients with PD.
12–14 Despite these findings, approximately 85% of individuals with PD do not have a family history of PD in a close relative.
10 Therefore, it has been suggested that markers for susceptibility to PD are needed.
6,10Several lines of evidence suggest that panic disorder is most compelling among DSM-IV anxiety disorders as a potential marker of susceptibility for increased risk of PD. Panic disorder has been associated with genetic causes in both the general population and in studies of genetic mutation in PD. A recent meta-analysis in the general population concluded that panic disorder aggregates in families, with an average odds ratio (OR) of 5.0, 95% confidence interval [CI]: 3.0–8.2, and an aggregate risk of 10.0% in relatives of panic disorder probands, as compared with 2.1% in relatives of unaffected probands.
15 Furthermore, the results of the meta-analysis suggested that the major source of familial risk is genetic. Panic disturbances have also been reported in both familial and sporadic cases of PD, with
LRRK2 and
Parkin mutations the most common monogenetic causes of PD. Two of the most common monogenetic causes of PD,
LRRK2 and
Parkin, have reported panic in both familial and sporadic cases with mutations in these genes.
16–18 Also, in previous work, we showed that patients with panic disorder and PD were distinguished by an earlier age at onset of PD and more frequent motor complications.
5 Panic-type phenomena are also associated with dopaminergic treatment and motor fluctuations.
19–22 Finally, both panic-type anxiety and PD have been associated with locus coeruleus pathology and noradrenergic dysfunction;
19,23,24 these findings indicate biologic plausibility for panic symptoms as a susceptibility marker for PD.
Evidence for a shared genetic predisposition between panic disorder and PD would require a higher prevalence of panic disorder in the first-degree relatives of individuals with PD who have panic disorder, in contrast to PD subjects without panic disorder. Accordingly, this study examined whether panic-type anxiety aggregates in the families of PD patients who have panic disorder (PD-PANIC) as compared to PD patients without any anxiety disorders (PD-NA).
METHOD
Subjects
Participants were adults with idiopathic PD recruited from movement-disorder practices in the greater Baltimore area and their first-degree relatives. Probands diagnosed with PD using U.K. Brain Bank Criteria
25 were selected consecutively from one of two cohorts: 1) Methods of Optimal Depression Detection in PD (MOOD-PD);
5 or 2) the Johns Hopkins Parkinson's Disease Research Center's (PDRC) Longitudinal Study of PD.
26 In each protocol, psychiatric diagnoses were determined by psychiatrists using the Structured Clinical Interview for Diagnosis (SCID) for DSM-IV Axis I Disorders, Research Version, Non-Patient Edition
27 as part of a comprehensive psychiatric evaluation. Recruitment started with subjects most recently enrolled in MOOD-PD; when that population was exhausted, the most recently enrolled subjects from the PDRC were recruited. Case probands with PD and panic disorder with or without agoraphobia (PD-PANIC; N=30) and a comparison group of non-anxious PD probands (PD-NA; N=56), defined as having no history of any anxiety disorder, no current mood disorder or major psychotic disorder, were identified. Individuals with Mini-Mental State Exam (MMSE) scores <24 were excluded. The Unified Parkinson's Disease Rating Scale (UPDRS) Motor
28 and Complications of Therapy subscores and Hoehn and Yahr Stage (HY)
29 were rated by movement-disorder specialists. Total
L-dopa equivalent daily dose
30 and Motor subtypes
31 were determined.
Probands were contacted by phone regarding their willingness to participate. Consenting probands were asked to act as representatives for their first-degree relatives by collecting contact information for these individuals. Each relative was mailed a description of the study and a postcard with return postage indicating consent to be contacted. Informed consent by relatives for participation in the study was obtained via telephone. A family was excluded when only the proband consented to participate; that is, when no relatives consented. The final sample included 37 probands and 222 first-degree relatives (20 PD-PANIC probands and 114 relatives and 17 PD-NA probands and 108 relatives).
Diagnostic Procedures for First-Degree Relatives
After informed consent was obtained, evaluations to determine panic status in relatives were conducted by phone, using the Panic Disorder module from the SCID.
27 When relatives were deceased, unreachable, or unwilling to participate; informant “proxy” interviews were conducted by interviewing another family member or spouse. For proxy interviews, panic status was assessed using the Family Informant Schedule and Criteria (FISC) for panic disorder.
32 Children (N=2, one in each group) below the age of 18 were evaluated by parental proxy. All interviews were conducted by a trained research coordinator.
Panic disorder was diagnosed in relatives who met DSM-IV criteria for panic disorder as follows: recurrent unexpected panic attacks (discrete period of intense fear with four or more of the following: palpitations, sweating, shaking, shortness of breath, feeling of choking, chest discomfort, nausea, feeling faint, fear of losing control, fear of dying, derealization, paresthesias, chills, or hot flashes), where at least one of the attacks has been followed by 1 month (or more) of one (or more) of persistent concern about having additional attacks, worry about the implications of the attack, or a change in behavior not due to the effects of a substance or better accounted for by another mental disorder. Relatives who endorsed recurrent unexpected panic attacks with two or more of the 13 symptoms of DSM-IV–defined panic attacks but did not otherwise meet behavioral or concern/worry criteria for disorder were considered to have a “panic-like” disturbance. “Panic-like” disturbances have been previously described in both the general population and in PD.
19,33 All SCID and FISC evaluations were reviewed by a psychiatrist who was blind to Case/Control status of the relative and categorized each relative as having panic disorder, panic-like phenomena, or no panic. Each relative completed a standardized form that inquired about demographic data and self-reported history of psychiatric illness and cognitive problems. These forms were mailed to each relative and reviewed by phone. Self-reported history of psychiatric illness and cognitive problems was captured as a yes-or-no response for depression, anxiety, hallucinations, memory issues, and other problems.
Data Analysis
Demographic and clinical variables in PD-PANIC and PD-NA probands and their first-degree relatives were compared, using t-tests and the chi-square statistic. Significance was set a priori at p<0.05.
The odds of panic disorder and panic-like phenomena in first-degree relatives were estimated using logistic regression by the method of generalized estimating equations, which accounts for within-family correlation among relatives.
34 Potential confounding factors (proband age, relative sex, current depressive disorder in PD-PANIC probands, and type of interview [direct versus proxy]) were controlled for in the regression models.
For analyses of DSM-IV panic disorder outcomes, relatives with panic-like disturbances were considered as Not Present and excluded from the numerator, but included in the denominator. For analyses with DSM-IV panic disorder and panic-like outcomes, relatives with panic-like disturbances were included in both the numerator and denominator. One relative with unknown panic status was excluded from both the numerator and the denominator in the analysis.
RESULTS
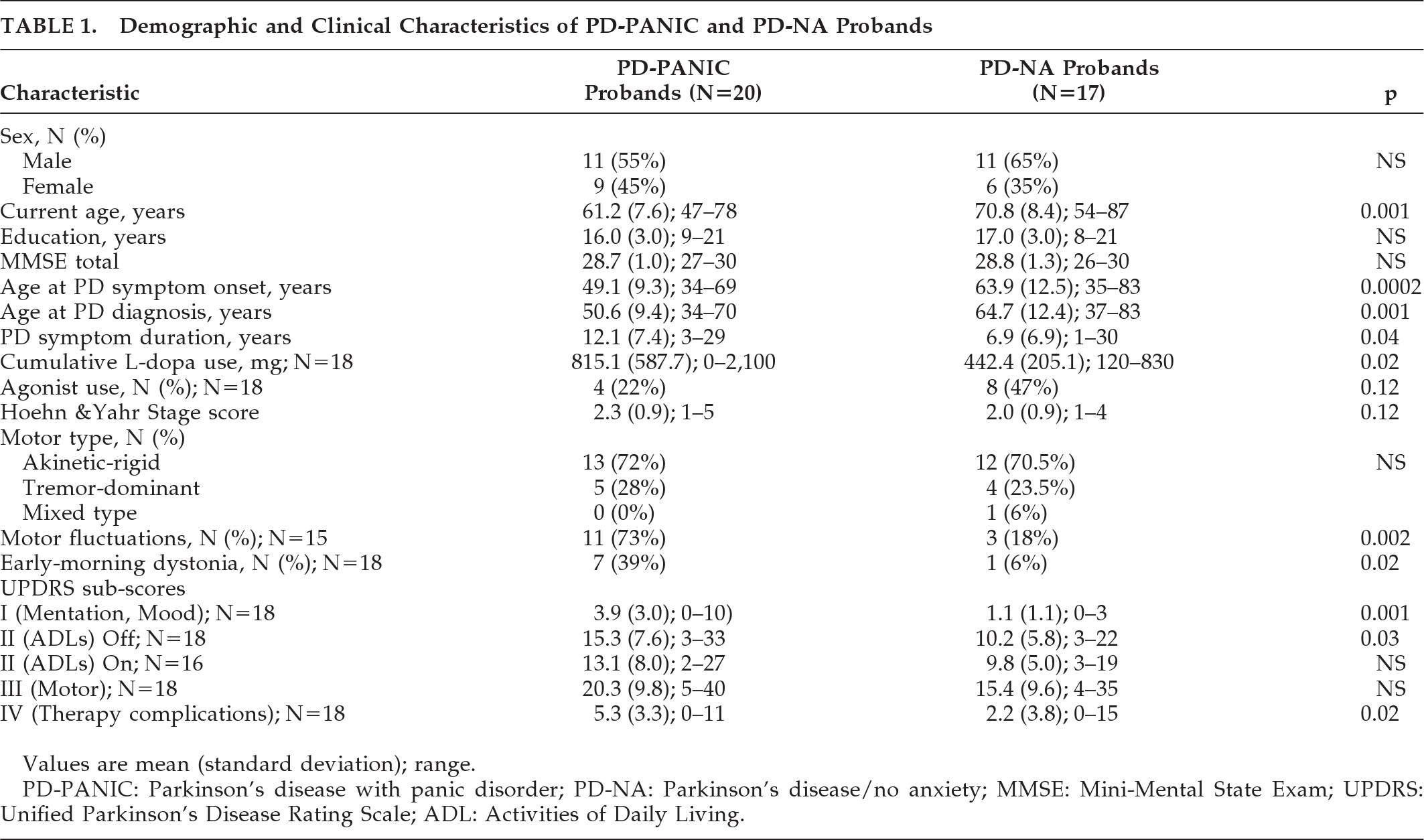
Table 1 shows the demographic and clinical features of the PD-PANIC and PD-NA probands. As compared with the PD-NA probands, PD-PANIC probands were significantly younger, with a longer duration of PD, higher
L-dopa doses, and greater activities of daily living (ADL) disability when in the “off” state, but comparable PD severity as measured by the Hoehn and Yahr stage and the UPDRS Motor subscore. Current depression was diagnosed in 50% of PD-PANIC probands (N=10) and, by definition, none of the PD-NA probands. Lifetime history of depression was diagnosed in 85% (N=17) of PD-PANIC probands and none of the PD-NA probands.
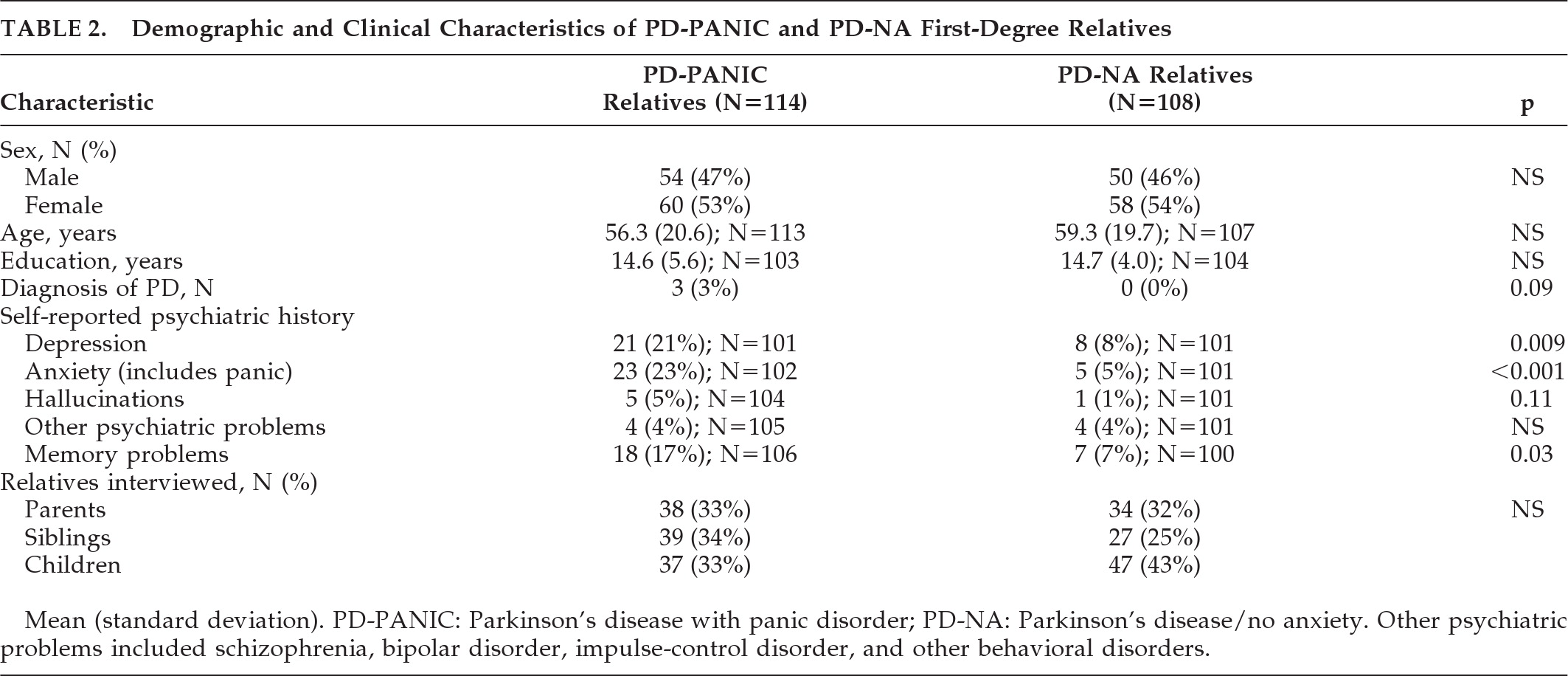
Table 2 compares the demographic features and self-reported history of psychiatric illness in first-degree relatives of PD-PANIC and PD-NA probands. The proportion of direct versus proxy interviews was similar between groups: direct interviews were conducted in 58% of the PD-PANIC relatives (N=67) and 46% of the PD-NA relatives (N=50). Proxy interviews were conducted in 46% of PD-PANIC relatives (N=50) and 54% of PD-NA relatives ((N=58; p<0.07). Panic status was determined by direct versus proxy interview 79% (N=15) versus 21% (N=4) of the time for PD-PANIC relatives and 100% (N=1) versus 0% (N=0) of the time for PD-NA relatives. Regardless of group, panic status was determined more often by direct interview 14% (16/116), as compared with 4% (4/106) by proxy (p=0.009) and in women (14%; (16/118) versus men (4%; 4/104; p=0.012). The mean number of PD-PANIC family members interviewed was 6.2 (standard deviation [SD]: 2.4); similar to the PD-NA families' average of 6.6 (2.1) relatives; NS. The number of siblings in the PD-PANIC and PD-NA families was comparable (2.1 [2.3] versus 1.6 [1.4]; NS). However, PD-PANIC probands had fewer children (2.1 [0.91]) than PD-NA (3.0 [1.2]; p=0.02). First-degree relatives were also similar in terms of age, sex, and type of relative interviewed.
As shown in
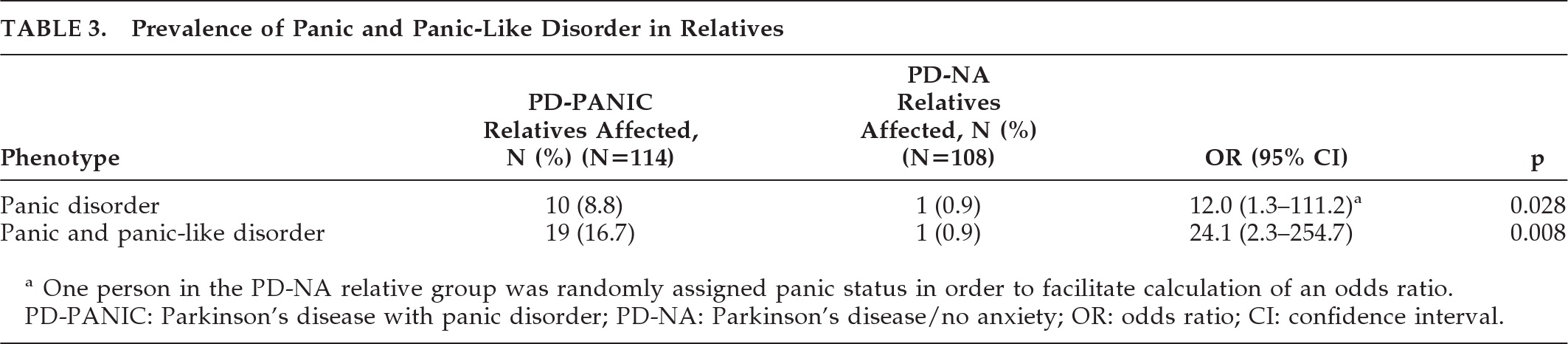
Table 3, the prevalence and odds of panic disorder and panic-like disorder are greater for relatives of PD-PANIC probands than PD-NA probands. Regression analyses (
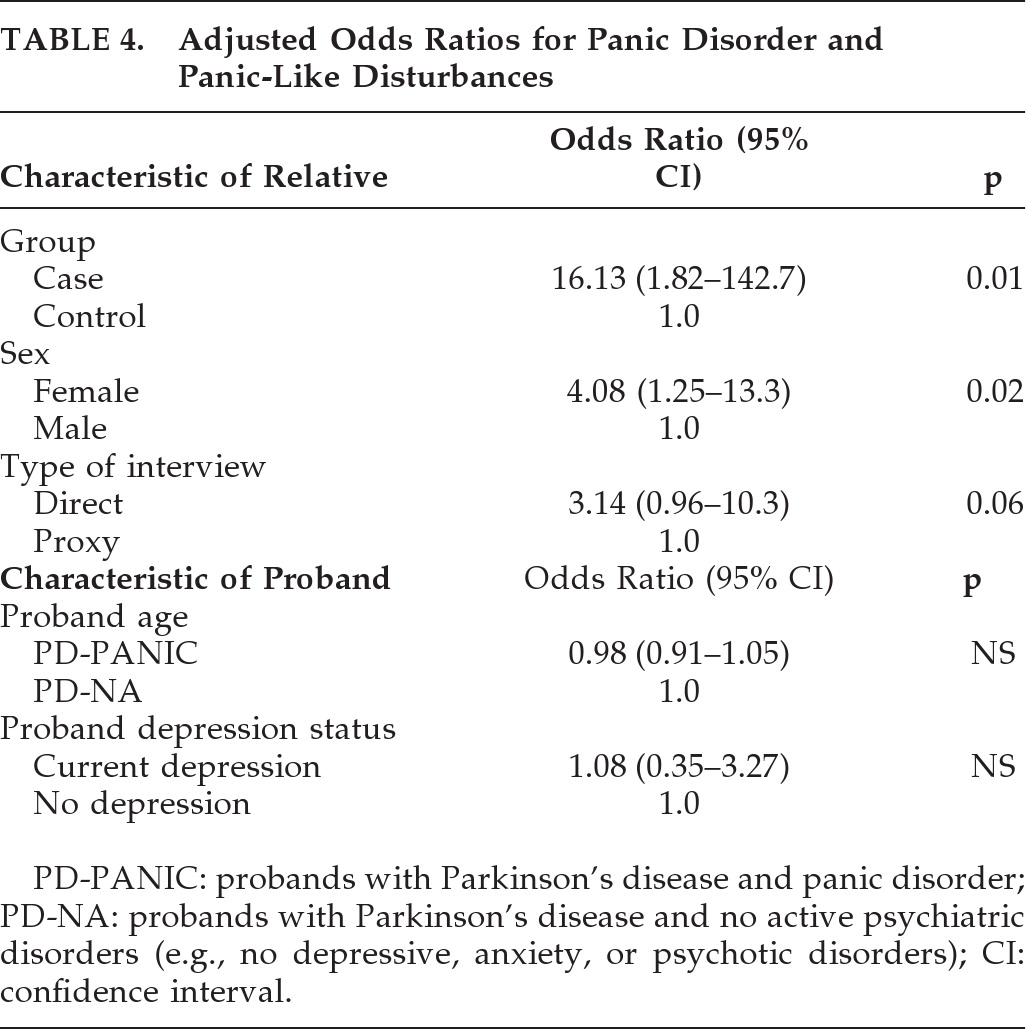
Table 4) showed that the significant group differences in prevalence rate persisted after controlling for potentially confounding variables: younger age of PD-PANIC probands, presence of current depressive disorder in probands, higher prevalence of panic phenotype in female relatives, and higher prevalence of the panic phenotype in directly-interviewed relatives. Among the 114 relatives of PD-PANIC probands, there were no significant differences in the frequency of a current panic disturbance in first-degree relatives of PD-PANIC with current depression (11/46) versus first-degree relatives of PD-PANIC without current depression (8/49; NS).
DISCUSSION
The greater severity of PD in patients with panic disturbances in our study, although incidental to our main hypothesis, suggests that panic may be a feature of a unique clinical subtype within PD. In comparisons of clinical features between the proband groups, PD onset occurred nearly 15 years earlier on average in the panic group. This is consistent with the study by Arabia et al.,
9 in which relatives of patients with earlier onset of PD had an increased risk of anxiety disorders as compared with those with later onset. Our study also showed that the PD patients with panic disturbances had longer PD duration, were more likely to experience motor and medication fluctuations, had much higher
L-dopa equivalent daily dose, and had more early-morning dystonia. Although our probands were divided into groups based exclusively on panic status and the absence of current psychiatric disturbance in the PD-NA group, the PD-PANIC group resembles the “younger-onset” clinical subgroup of PD.
35–38 Lewis et al.
35 and other studies that replicate their findings describe a subgroup of patients with earlier onset of PD, longer disease duration, higher
L-dopa use, and “greater potential to develop motor fluctuations.”
35–38 In general, idiopathic PD is thought to be both clinically and etiologically heterogeneous with genetic risks, being greatest in younger patients.
10 Taken together, the unique clinical features, earlier age at PD onset, and familial predisposition to panic suggests that the PD-PANIC probands may represent a unique subgroup of PD patients.
The results of this study also support the familial nature of DSM-IV panic disorder and “panic-like” disturbances in PD. In the first-degree relatives of PD patients with panic disorder, the lifetime prevalence of panic disorder and panic-like disturbances was more than 24 times greater than seen in first-degree relatives of PD patients without anxiety. Although heritability does not confirm a genetic etiology, our finding that both panic disorder and panic-like disturbances are familial is consistent with twin studies in the general population; these show that “panic-like” anxiety is on the same continuum of genetic liability as strictly defined DSM-IV panic disorder.
33 However, in the absence of non-PD controls with panic disorder, we cannot conclude that the increased odds of panic disorder in relatives of PD-PANIC probands is specific to PD. Nonetheless, these results provide evidence that panic-type anxiety is not simply a reaction to the disease burden of PD or the result of antiparkinsonian therapies.
The significantly younger age of the PD-panic probands, although, on one hand, supportive of greater PD pathology, is also a limitation, since the probands were not age-matched. Previous studies on motor complications of PD treatment report that earlier age at treatment and more severe disease stage are risk factors for complications of antiparkinsonian medications.
35,36 Therefore, motor complications may be overrepresented in PD-PANIC probands because of their younger age; however, we note that stage of disease was similar in the PD-PANIC and PD-NA groups. Although there is generally thought to be a lower prevalence of panic disorder in older than in younger subjects,
39 it is unlikely that our sample prevalence was affected because the primary outcome was panic status in relatives, for which there was no age difference. Our finding of an increased prevalence of panic in female relatives is consistent with a greater frequency of panic in women in the general population.
39We also note that we were unable to match by depression status in our proband groups. This limits the ability to detect the potential contribution of depression to the heritability of panic. In our sample and in others, the high lifetime comorbidity of anxiety and depression in PD (up to 92% in a previous one study),
4 makes it difficult to identify anxious individuals without a history of depressive disturbances and vice versa. When proband current depression status was included in the final regression model (
Table 4), the results suggest that heritability of panic is independent of current depression status in the proband. However, we were unable to control for the effect of a lifetime history of depression on panic since only three of the PD-PANIC probands had no lifetime history of depression. Mitigating this concern, however, is the finding from two studies in the general population that familial aggregation of panic is unaffected by comorbid mood and non-panic anxiety disorders.
15,40 In PD samples, the increased risk of depression in relatives appears to be independent from that of anxiety.
7,9 Whereas we also found increased prevalence of self-reported depressive disorders in PD-PANIC relatives, as compared with PD-NA relatives, this finding is likely affected by the exclusion of depressive disorders in the PD-NA probands.
An additional limitation is that not all relatives were directly interviewed. Data regarding deceased, unwilling, and unreachable relatives was obtained via proxy interview. Because many panic symptoms (e.g., derealization and paresthesias) are subjective and cannot be observed directly, proxy methods may underreport cases. Indeed, more panic and ‘panic-like’ cases were identified by direct interview than by proxy interview. Although this may be a source of ascertainment bias, we note that the proportion of direct and proxy interviews was similar across the PD-PANIC and PD-NA relative groups.
The finding that panic disorder and “panic-like” disturbances are familial in PD suggests that they may be genetically based and not simply accounted for by exposure to dopaminergic therapy or a reaction to motor impairment. The similarities between PD-PANIC probands and a previously-described subgroup of “younger-onset” PD patients
35–38 suggests that panic-type anxiety may be an additional clinical marker for earlier-onset PD and possibly an endophenotypic feature that co-segregates with relatives at increased risk for this PD variant. Given the implications of these findings and the high prevalence of panic disorder in patients with PD, routine screening for panic phenomena in patients with PD should be considered.
Acknowledgments
Authors report the following grants:
Gregory Pontone: Grants: National Institutes of Health, Forest Research Institute, Parkinson's Disease Foundation/Parkinson Study Group.
O. Joseph Bienvenu: Grant: National institutes of Health (K23 MH64543), Contracts (research): APA.
Kung-Yee Liang, Gerald Nestadt, Peter V. Rabins: Grants: National institutes of Health
Peter V. Rabins: Expert Testimony: Janssen Pharmaceuticals.
L. Marsh: Advisory Boards, National Parkinson Foundation, American Parkinson's Disease Association; Grants, National Institutes of Health, Forest Research Institute, Eli Lilly, Michael J Fox Foundation; Employment: Michael E. DeBakey Veterans Affairs Medical Center, Baylor College of Medicine; Royalties: Taylor and Francis/Informa.
This work was supported by: NIH grants R01-MH069666, the Morris K. Udall Parkinson's Disease Research Center of Excellence at Johns Hopkins (NIH-P50-NS-58377) and the Age-Related Cognitive Disorders Training Grant (NIH-5T32-AG-027668-02) to J.R. Williams, the Parkinson's Disease Foundation/Parkinson Study Group Mentored Clinical Research Award (Gregory M. Pontone, M.D.), and the Donna Jeanne Gault Baumann Fund.
The views expressed in this manuscript do not necessarily represent the views of the Food and Drug Administration or the United States.