Topiramate (Topamax), a drug approved for epilepsy and migraine, has shown promise in treating alcohol dependence in a randomized, double-blind study published in the October 10 Journal of the American Medical Association (JAMA).
Adult patients who met DSM-IV criteria for alcohol dependence were enrolled in the study; those with psychiatric comorbidities (except alcohol, nicotine, or caffeine dependence) or recent substance abuse history were excluded. Half of the participants received topiramate and half received placebo for 14 weeks. All participants received a weekly brief behavioral treatment, delivered in about 15 minutes by trained personnel. The intervention provided motivational support and tools for adhering to medication treatment. A few participants attended Alcoholics Anonymous meetings during the study.
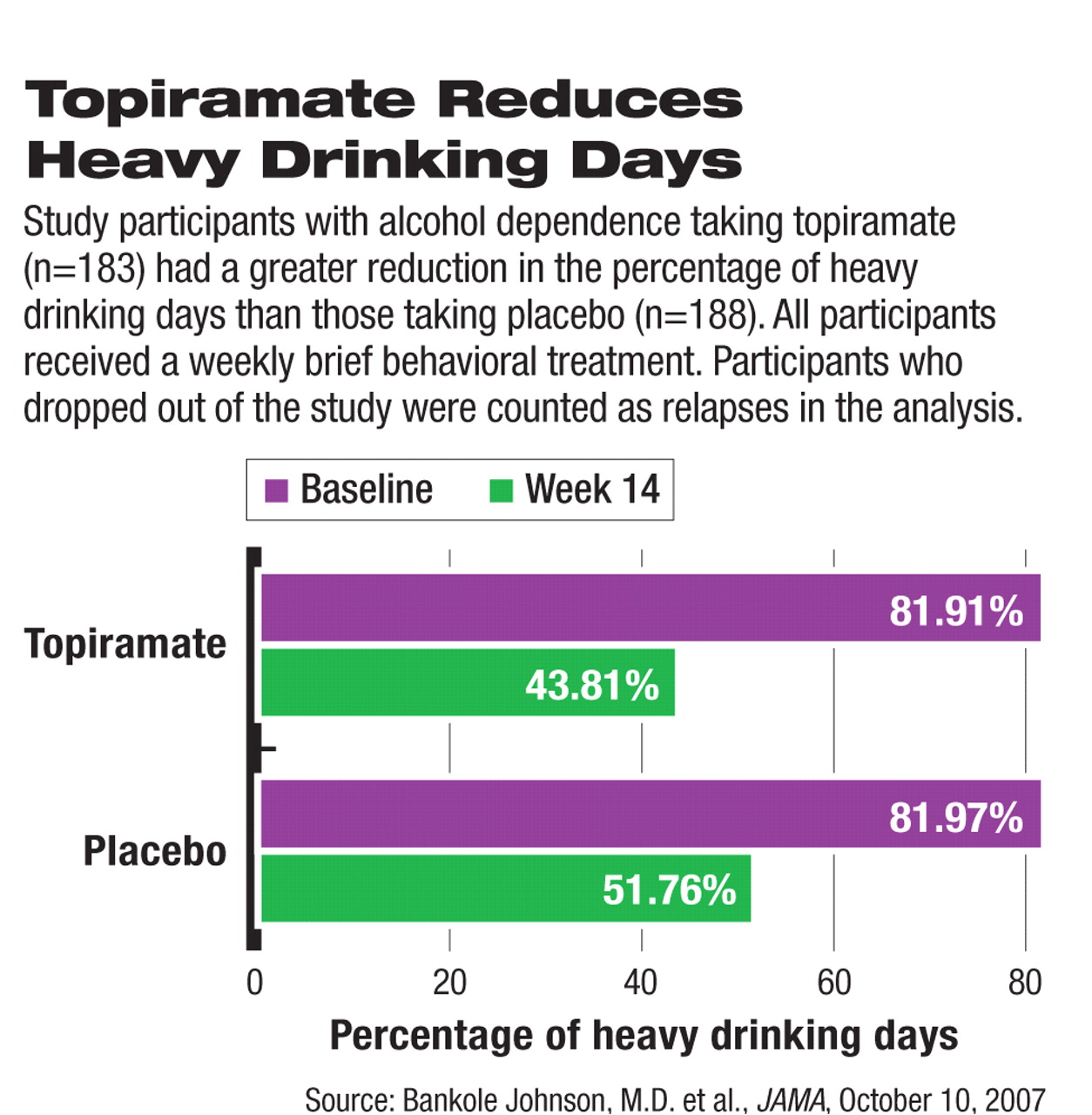
At the end of week 14, self-reported heavy-drinking days dropped from about 82 percent to 44 percent in the topiramate group and from 82 percent to 52 percent in the placebo group. The reduction was significantly higher in the topiramate group than in the placebo group, regardless of whether dropout patients were included in the calculation. The statistical significance between the two groups was achieved at week 4 and persisted through the end of week 14.
The self-reported drinking reduction was corroborated by the participants' plasma γ-glutamyltransferase, a liver enzyme indicating recent drinking, which also showed statistically significant difference between the topiramate and placebo groups. The rates of achieving at least 28 days of no heavy drinking or continuous abstinence were also statistically significantly higher in the topiramate group.
The dosage of topiramate tested in this study was 50 mg/day to 300 mg/day titrated over a six-week period. The mechanism of topiramate's effect may involve a range of activities on the GABA and glutamate receptors, the authors suggested.
Study participants in the topiramate group experienced more adverse effects than did the placebo group. The most commonly reported side effects that were significantly different between the treatment groups included tingling or numbing sensation in the skin (50.8 percent in the topiramate group versus 10.6 percent in the placebo group), change in taste (23 percent versus 4.8 percent), loss of appetite (19.7 percent versus 6.9 percent), and difficulty concentrating or paying attention (14.8 percent versus 3.2 percent).
Of note, 67 participants dropped out of the topiramate group before study completion; 34 did so because of adverse events. In the placebo group, 41 dropped out, including six because of adverse events. The authors recommended a slower titration over eight weeks, an approach that, in a study published in the May 17, 2003, The Lancet, achieved a participant retention rate similar to placebo.
The study was funded by Ortho-McNeil Janssen, a subsidiary of Johnson and Johnson. Ortho-McNeil Neurologics, also under Johnson and Johnson, manufactures topiramate.
“Our study shows that topiramate helped patients with alcohol dependence get better even during times of heavy drinking,” Bankole Johnson, M.D., Ph.D., chair of the Department of Psychiatric Medicine at the University of Virginia and the lead author of the study, told Psychiatric News. “And it shows that a brief, simple, 15-minute behavioral intervention can be effective. It is easy to provide this intervention in the primary care setting by doctors or nurses, which will increase patients' access to the treatment they need for alcohol dependence.” Johnson is a member of the APA Council on Addiction Psychiatry.
The brief intervention manual used in the study can be obtained from Johnson or found in Handbook of Clinical Alcoholism Treatment, of which Johnson is a co-author.
Currently, oral and injectable naltrexone and acamprosate are approved pharmacological treatments for alcohol dependence in addition to the old drug disulfiram.
“While topiramate is not currently approved for the treatment of alcohol dependence, this is an important study to show the drug's potential effect in decreasing alcohol use in persons with alcohol dependence,” said Eric Strain, M.D., professor and section head of JHB Psychiatry Substance Abuse Programs at Johns Hopkins University School of Medicine and chair of APA's Council on Addiction Psychiatry. He emphasized that available pharmacotherapy is effective for treating patients with alcohol dependence and deserves a place in the treatment plan.
“Psychiatrists should be familiar with these approved medications and knowledgeable about new medications that may soon become available to help patients with alcohol-use disorders.”
An abstract of “Topiramate for Treating Alcohol Dependence” is posted at<jama.ama-assn.org/cgi/content/abstract/298/14/1641>. An abstract of “Oral Topiramate for Treatment of Alcohol Dependence: A Randomised Controlled Trial” is posted at<www.thelancet.com/journals/lancet/article/PIIS0140673603133703/abstract>.▪

