In patients with attention-deficit/hyperactivity disorder (ADHD) aged 3 to 20, the use of stimulants is linked to a small increase in the risk of visiting the emergency department (ED) and physicians' offices for cardiac symptoms, but not to the incidence rates of cardiac deaths and hospitalizations, according to a large retrospective study by researchers at the University of Florida.
The authors used a large dataset, the Florida state Medicaid claims database more than a 10-year period, to conduct their analyses. From July 1994 to June 2004, more than 55,000 patients between the ages of 3 and 20 enrolled in the Medicaid program had a new ADHD diagnosis or new stimulant prescription claim, defined as no previous diagnosis or stimulant claims for six months before the first diagnosis appeared in the system. Of the patients with ADHD, 32,807 had prescription claims for stimulants in the database, namely for methylphenidate, amphetamine, dexamphetamine, or pemoline; this translated to a total of 42,612 person-years of stimulant use. The mean age of the first ADHD diagnosis or stimulant prescription was 8 years.
During the study period, the all-cause death rate was very low, including five patients who died of circulatory system disease (translating to an incidence rate of 4 per 100,000 person-years). No one died while on stimulants. The incidence rate of hospital admission for cardiac reasons was 21.6 per 100,000 person-years. Both rates were too low for statistical comparisons between those who took stimulants and those who did not. The authors concluded that the risk of cardiac-related death or hospitalization is small and similar to the national average for this population.
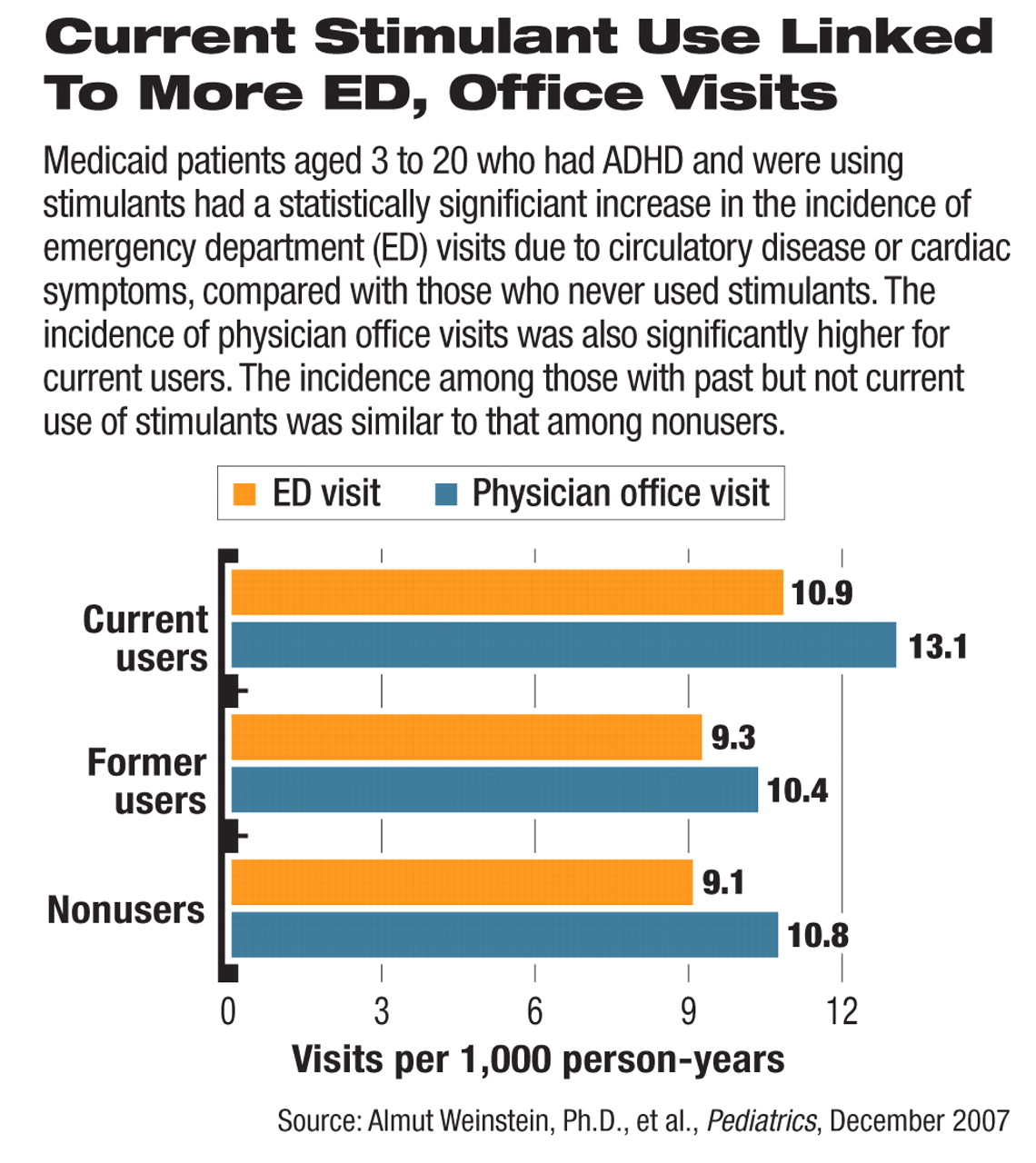
The incidence for ED visits because of cardiac symptoms was 10.9 per 1,000 person-years in those who were on stimulants. Compared with the rate in those who had not used stimulants (9.1 per 1,000 person-years), the difference was statistically significantly higher by 20 percent (hazard ratio: 1.20; 95 percent confidence interval: 1.04 to 1.38). The authors found that patients who had taken stimulants before but were not currently taking them had an incidence of heart-related ED visits similar to those who had never filed a claim for these medications.
Similarly, the incidence rate of physician office visits for heart-related symptoms or problems was 21 percent higher in the current stimulant users compared with nonusers (13.1 and 10.8 visits per 1,000 person-years, respectively), a statistically significant difference. Patients who had taken stimulants before did not differ significantly from nonusers.
“Many psychiatrists have already known that the cardiac side effects of stimulants are not a major concern in most patients,” said David Mrazek, M.D., chair of APA's Council on Children, Adolescents, and Their Families and chair of the psychiatry and psychology departments at the Mayo Clinic. “The strong negative findings in terms of severe events and mortality are reassuring.”
He commented that the study design, which was observational, limited the interpretation of the positive findings regarding the increased incidence of ED and office visits. “The relative nonserious nature of these events also provides the reassurance that most patients taking stimulants are not in severe, obvious danger,” he said.
The study was funded by the Florida Department of Health, the Agency for Healthcare Administration, the Medicaid program, and the University of Florida and was published in the December 2007 Pediatrics.
“The small incidences of severe events and the comparably small [hazard ratio] for cardiac ED visits allow the suggestion that the risk of stimulants is more subtle than the review of case reports may suggest,” the authors wrote. They also cautioned that their findings are limited to the study duration and do not rule out the possible risk of developing heart disease with long-term ADHD use. To help resolve the controversy surrounding the heart safety of ADHD drugs, the Food and Drug Administration and Agency for Healthcare Research and Quality are currently collaborating on a large study to investigate the cardiovascular risks of ADHD drugs in children as well as adults (Psychiatric News, October 19, 2007).
An abstract of “Cardiac Safety of Central Nervous System Stimulants in Children and Adolescents With Attention-Deficit/Hyperactivity Disorder” is posted at<pediatrics.aappublications.org/cgi/content/abstract/120/6/e1494>.▪

