Pharmaceutical companies are racing to develop new classes of drugs to stop or reverse the course of Alzheimer's disease, but the forecast for many drug candidates remains somewhat murky.
Alzheimer's affects an estimated 5 million people in the United States, and the prevalence doubles for every five years beyond the age of 65, according to the Centers for Disease Control and Prevention. As life expectancy continues to rise and the elderly population grows faster than other age segments, Alzheimer's looms as one of the largest public health problems. By 2050, the worldwide prevalence of Alzheimer's is expected to quadruple.
Currently, three cholinesterase inhibitors (donepezil, rivastigmine, and galantamine) and one NMDA (N-methyl-D-aspartic acid) glutamate receptor antagonist (memantine) are approved by the FDA for the treatment of Alzheimer's. These drugs slow disease progression with modest efficacy, but none is able to stop or reverse the mental decline.
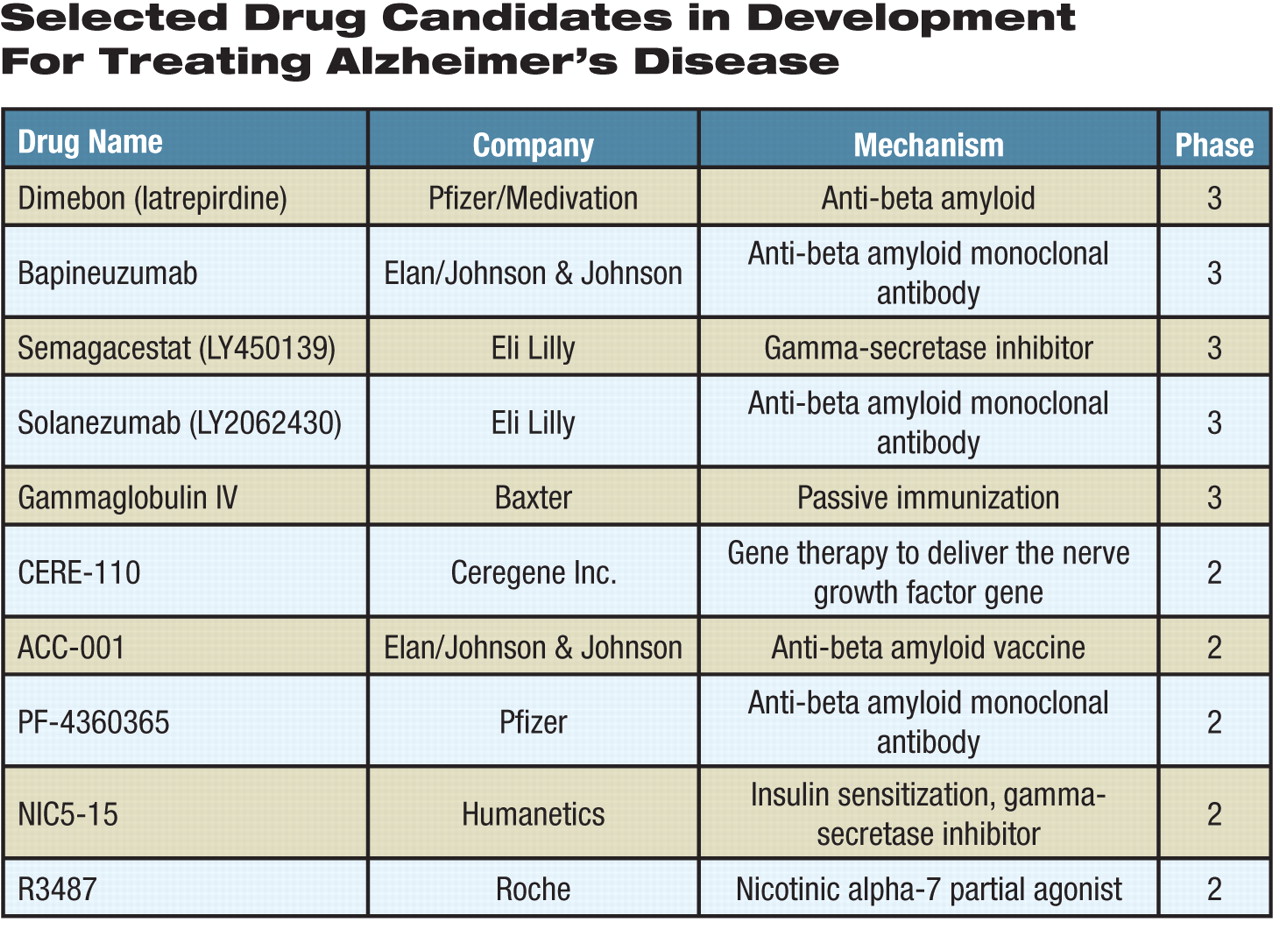
In recent years, several promising new drug candidates have been eagerly pursued by drug companies in research and development, and several have recently moved into phase 3 clinical trials (see table). Many molecules in the development pipeline, particularly biologics, target the formation or aggregation of beta amyloid, a peptide that is widely considered by scientists to play a key role in Alzheimer's pathology, particularly in the formation of protein plaques throughout the brain.
One of the drug candidates in phase 3 clinical trials is bapineuzumab, a monoclonal antibody that binds to beta amyloid and clears it from the brain. Johnson and Johnson recently bought the bapineuzumab development program from Elan, an Irish company, for $1.5 billion. However, doubts about bapineuzumab's efficacy emerged after it failed to achieve the primary endpoint in a phase 2 trial. Previous clinical-trial data also raised a safety concern about vasogenic edema, or accumulation of fluid in brain tissues, in some patients. Nevertheless, the trial suggested that the drug may be efficacious in a subset of patients who do not carry the apolipoprotein E4 (ApoE4) allele. The allele increases the risk for developing Alzheimer's, but not all Alzheimer's patients are carriers.
Eli Lilly also has an investigational monoclonal antibody known as solanezumab, in phase 3 clinical development. The antibody neutralizes beta amyloid.
Dimebon (also known as latrepirdine) is another promising candidate currently being tested in phase 3 clinical trials. The drug is a small molecule that was used for years in Russia as an antihistamine under the name of dimebolin. In a phase 2 clinical trial whose results were published in the July 19, 2008, The Lancet, patients with mild to moderate Alzheimer's who received dimebon for 26 weeks showed statistically significant benefits in cognitive functions compared with those who received placebo. The drug is being developed by Medivation and Pfizer.
Surprisingly, dimebon was shown to increase the level of beta amyloid in the brain, according to animal research studies released at the International Conference on Alzheimer's Disease (ICAD) in July in Vienna. The finding calls into question the pharmacological rationale and effectiveness of blocking beta amyloid in treating Alzheimer's.
At the ICAD, Abbott announced that it had terminated the development of one investigational drug for Alzheimer's. Two other molecules that interfere with the beta-amyloid formation process—tarenflurbil, developed by Myriad Pharmaceuticals, and tramiprosate, developed by Neurochem—have recently failed clinical trials. Nevertheless, beta amyloid remains the main target for development research.
Meanwhile, molecular targets other than beta amyloid are being explored by small and large pharmaceutical companies in search of magic bullets against Alzheimer's. Eli Lilly's semagacestat inhibits gamma-secretase and is in two phase 3 trials. NIC5-15, a plant-derived substance tested by Humanetics in phase 2 clinical development, is another gamma-secretase inhibitor with insulin-sensitizing effects. Gamma-secretase is involved in the production of beta amyloid, and its inhibition may decrease the production of beta amyloid.
Roche is testing R3487, a molecule it acquired from Memory Pharmaceuticals, as a treatment for both Alzheimer's and schizophrenia. The drug is a nicotinic alpha-7 partial agonist.
Baxter Healthcare and the National Institutes of Health are cosponsoring a phase 3 trial in Alzheimer's patients on the efficacy of an intravenous immunoglobulin product (gammaglobulin IV) it manufactures. In a retrospective, case-control study published in the July 21 Neurology, a history of intravenous immunoglobulin use was associated with a lower risk of developing Alzheimer's in four years. It has been speculated that passive immunization with a mixture of human immunoglobulins, or antibodies, derived from human plasma may remove beta amyloid from the body. Other vaccines targeting beta amyloid are also being investigated by pharmaceutical companies.
An abstract of “Effect of Dimebon on Cognition, Activities of Daily Living, Behaviour, and Global Function in Patients With Mild-to-Moderate Alzheimer's Disease: A Randomised, Double-Blind, Placebo-Controlled Study” is posted at<www.thelancet.com/journals/lancet/article/PIIS0140-6736(08)61074-0/abstract>. An abstract of “IV Immunoglobulin Is Associated With a Reduced Risk of Alzheimer Disease and Related Disorders” is posted at<www.neurology.org/cgi/content/abstract/73/3/180>.▪