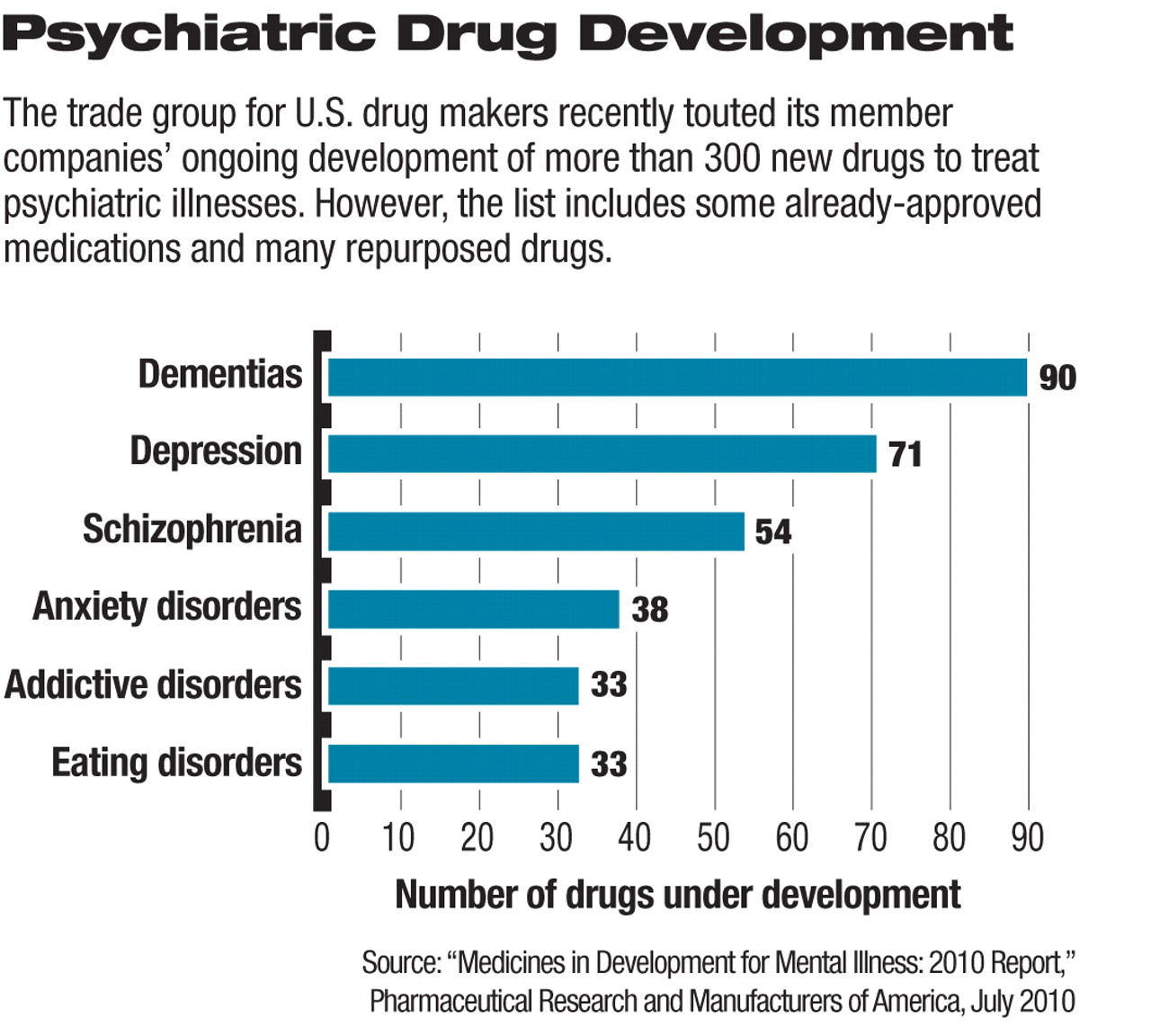
The more than 300 new drugs under development to treat a variety of mental illnesses represent an increase in the tally of such new products in recent years, according to a drug-industry report. However, many of these medications are either new applications for existing medications or “me too” drugs that use existing mechanisms of action in slightly modified forms.
U.S. pharmaceutical research and biotechnology companies have 313 medications in development to treat the nearly 60 million American adults suffering from various types of mental illness, according to a July report by the Pharmaceutical Research and Manufacturers of America (PhRMA), the drug industry's trade group. The largest numbers of new products under study are designed to treat dementia (90), depression (71), schizophrenia (54), and anxiety disorders (38).
“Over the past half century, pharmaceutical research has helped transform mental illnesses from misunderstood causes of shame and fear into often highly treatable conditions,” said David Weadon, M.D., senior vice president for scientific and regulatory affairs at PhRMA, in the report.
The large number of potential drugs in development does not, however, mean that major treatment breakthroughs are on the way for patients with most types of mental illness, said Jeffrey Lieberman, M.D., chair of APA's Council on Research and Quality Care, in an interview with Psychiatric News.
While more-effective medical treatments are needed for psychiatric conditions, many of the drugs detailed in the PhRMA report are new formulations of existing compounds or new compounds that use existing mechanisms of action, he said. The list also includes drugs that have already received Food and Drug Administration approval.
“I wouldn't say that the pipeline for new treatments is exactly full right now,” Lieberman stated.
Lieberman described the new medications under development that use known mechanisms of action as “me too” drugs, because they are designed to compete with existing treatments instead of offering new approaches to address the underlying psychiatric condition.
The list of psychiatric medications under study includes existing medications for which drug makers are looking for new uses than those for which they were previously approved. One example is a clinical trial on the ability of the sleep medication Lunesta to treat anxiety.
“There is nothing really new there,” said Sarah Terry, president of Life Science Analytics, a market research firm, about mental health drugs under development.
Her firm has concluded that only about 31 truly new psychiatric medications are under development to treat anxiety and depression, for example.
One area of mental health care in which truly new treatments are emerging, according to Decision Resources, another market research company, is in treating major depressive disorder. At least four companies worldwide are developing corticotropin-releasing factor 1 (CRF1) receptor antagonists, whose mechanism of action is a departure from the classic monoamine approach that most existing antidepressants use. However, no CRF1 agent has advanced beyond phase 2 clinical trials.
Lieberman applauded the fact that a large number of studies focused on treatments for dementia are under way. These likely stem from recent advances in neurobiological research and may advance treatment beyond the capabilities of approved medications, which cannot arrest—much less reverse—dementia-related conditions.
Similar medication advances are most sorely needed for schizophrenia, autism, and substance use disorders, Lieberman noted.
Substance use disorders are another category of mental illness in which additional medication options are especially needed. Mental health advocates in Congress are aware of the dearth of such medications, and the need to develop additional ones was the focus of a June congressional hearing (Psychiatric News, August 6). Speakers testifying at the hearing said that many drug companies have begun to move away from the development of treatments for psychiatric disorders, such as substance abuse, because of a variety of market pressures, including the high investment cost of developing new treatments.
Research funding has moved away from psychiatric treatment development, specifically, because it is viewed as having the potential for both “high gain and high risk.” Such financial concerns take on great weight in light of the average $1.3 billion research and development cost and up to 15 years it takes to bring new medicines to patients. Additionally, psychiatric illnesses frequently lack the types of “hard, objective end points,” which indicate treatment success in other areas of medicine; instead, their outcomes are often measured through more subjective patient ratings, Lieberman noted.