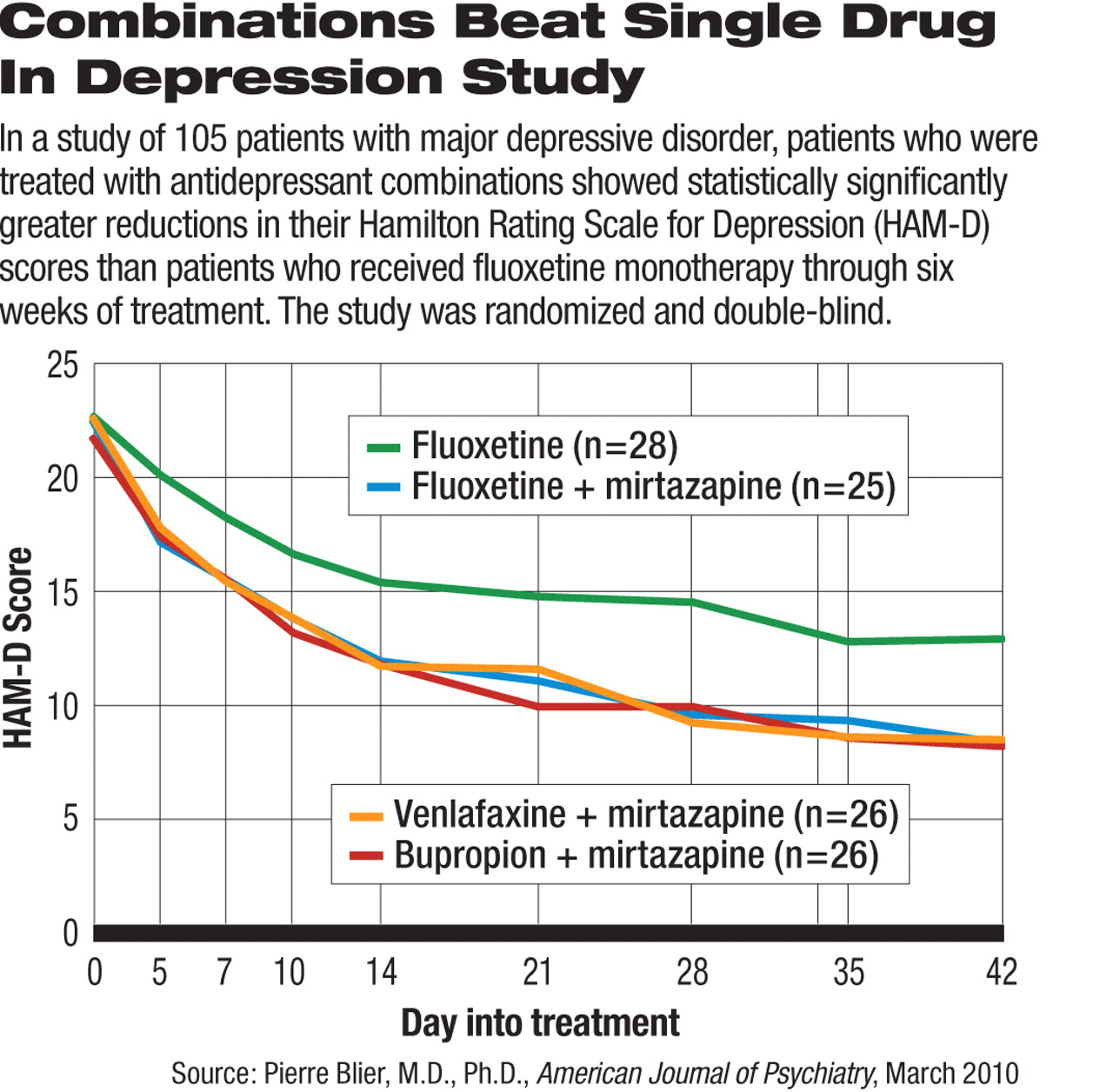
The use of two-drug combinations may be more effective than monotherapy to induce remission in patients with major depressive disorder, according to a study published in the March American Journal of Psychiatry.
In previous clinical trials of various antidepressants, the rate of remission consistently hovered around one-third after an adequate trial of monotherapy for four to eight weeks. The majority of patients who do not achieve remission on a single antidepressant are generally switched to another antidepressant or receive an add-on therapy. In the new study, Pierre Blier, M.D., Ph.D., at the University of Ottawa Institute of Mental Health Research, and colleagues conducted a randomized, double-blind study to test whether it would make sense to start patients on a two-drug combination therapy rather than undergo monotherapy for weeks.
One hundred and five patients with major depressive disorder (more than half had recurrent depressive episodes) were first taken off all psychotropic drugs for a washout period, then randomized to one of four antidepressant treatments: fluoxetine plus placebo (n=28), mirtazapine plus fluoxetine (n=25), mirtazapine plus venlafaxine (n=26), and mirtazapine plus bupropion (n=26). The washout period lasted at least five half-lives of the psychotropic medications taken by the patient before the study to ensure complete clearance from the body.
After six weeks of treatment, 45 percent of patients on mirtazapine plus bupropion, 52 percent on mirtazapine plus fluoxetine, and 58 percent on mirtazapine and venlafaxine reached remission, defined as a sustained score on the Hamilton Rating Scale for Depression (HAM-D) of no more than 7. These remission rates did not differ significantly from each other, but were all significantly higher than the 25 percent remission rate in the fluoxetine monotherapy group.
In contrast, the rate of response, defined as a sustained reduction of HAM-D score by at least half from baseline, did not show a statistically significant difference among the treatment groups. At the end of six weeks, the response rates were fluoxetine monotherapy: 54 percent; mirtazapine plus bupropion: 65 percent; mirtazapine plus fluoxetine: 68 percent; and mirtazapine plus venlafaxine: 73 percent.
At the end of the first six weeks of treatment, 66 patients with marked symptom improvement, as indicated by a Montgomery-Asberg Depression Rating Scale (MADRS) score of no more than 12, continued on a six-month additional phase of the study. The study remained double-blind while these patients were given either fluoxetine or mirtazapine monotherapy. In other words, those who were on combined therapies had one of the drugs dropped. About 40 percent of these patients relapsed within six months with no significant difference between fluoxetine and mirtazapine monotherapies.
Before completing the initial six-week treatment, 16 (15 percent) of the patients dropped out of the study in similar proportions in all four groups. Patients on mirtazapine gained significantly more weight from baseline compared with patients on fluoxetine only. No suicide attempt occurred in any of the 105 patients, and the treatments showed similar tolerability.
The findings of this study, the authors concluded, add to “mounting evidence that combination therapy from treatment initiation provides superior clinical effectiveness.”
The study was conducted at the University of Florida and University of Ottawa and supported by Organon, the maker of mirtazapine. Organon was recently acquired by Schering-Plough.