Second-generation antipsychotics have diffused rapidly among antipsychotic medication users and have generally been regarded as a first-line treatment for schizophrenia (
1,
2,
3,
4 ). Additional indications by the Food and Drug Administration (FDA) for use in bipolar disorder have increased the rate of antipsychotic use, as have significant levels of off-label use and use among youths (
5,
6,
7,
8 ). Antipsychotics have greater off-label use than many other therapeutic classes, and these uses may not be well supported by scientific evidence (
9 ).
Although spending on psychotropic medications is increasing at a higher annual rate than is spending on other medications (17.1% versus 12.1%) (
10,
11 ), psychotropic drugs may still be underused in mental health care (
12 ) because of barriers to treatment, such as cost and stigma. Increases in antipsychotic medication use are justifiable if the population with conditions appropriately treated with antipsychotics, such as schizophrenia and bipolar disorder, are undertreated and if antipsychotic medication treatment is cost-effective (
13 ) compared with other alternatives. This implies that second-generation antipsychotics may not have to "pay for themselves" in terms of providing an offset in lower total health care costs (
14 ), but they should provide greater benefits per dollar spent than older, less expensive antipsychotics or other treatments.
Although there is significant variation in the benefit from drug products across individuals (
15 ), in theory, drugs that provide greater value should diffuse much more quickly than other drugs. However, this is not always the case. Treatment decisions are made under a veil of uncertainty and market imperfections, and they are subject to a host of influences on prescribers: prescribers are never sure a priori which products will work best for any individual, and information on side effects is often recognized over time (as has been the case with significant product withdrawals, such as Vioxx). Diffusion of specific medications into treatment can be improved with a larger evidence base (
16 ) on which to examine whether the growth of particular products is indeed rational.
The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) was an effort to examine whether the rapid diffusion of second-generation antipsychotics is well informed. To do so, the study examined the effectiveness and tolerability of four second-generation antipsychotics (olanzapine, quetiapine, risperidone, and ziprasidone) and a single proxy for first-generation antipsychotics (perphenazine) (
17 ). The CATIE cost-effectiveness analysis (
1 ) found that although the outcomes expressed in quality-adjusted life years were similar across all five drugs, the total health care costs for patients randomly assigned to receive the first-generation antipsychotic, perphenazine, were substantially lower than that for those who received one of the second-generation antipsychotics. A recent study in the United Kingdom comparing first- and second-generation antipsychotics in a sample of previously treated individuals with schizophrenia found lower costs and better outcomes for the older medications (
18 ). These results may indicate that the widespread use of second-generation antipsychotics over first-generation alternatives may not be the best use of limited resources.
Although the diffusion of second-generation antipsychotics has been described in the literature (
2,
3,
12 ), the method by which these gains have been achieved has not been explored. There are two primary means of achieving market growth for pharmaceutical products: increases in the number of medication users or increases in dose per user. These two mechanisms are not mutually exclusive. It is unknown which one is driving the current growth in antipsychotic medications, although one recent study using Medical Expenditure Panel Survey (MEPS) data noted that just over one-third of the increase in the larger class of psychotropic medication was due to new users, whereas two-thirds of the increase was due to greater spending per user (
6 ). In addition, much of our knowledge about antipsychotic medication use comes from Medicaid populations (
2,
19 ), with little information available on trends in the use of antipsychotics from privately insured and uninsured populations.
This study sought to fill these gaps in the literature by examining the prevalence of antipsychotic medication use in the general population and the characteristics of antipsychotic users, including the rate of second-generation antipsychotic use and the level of use per user.
Methods
We used data from the Medical Expenditure Panel Survey (MEPS) from 1996–1997 and 2004–2005 to examine the characteristics of antipsychotic users in each time period as well as the rate of antipsychotic use in key subpopulations. The MEPS is a rich overlapping panel data set with a randomly selected annual sample of approximately 23,000–35,000 noninstitutionalized U.S. civilians and detailed information on health care services used in each survey year, insurance coverage, and expenditures by source (
20 ). Response rates ranged from 60% to 70% for the annual cohorts examined here, and survey weights were adjusted for nonresponse (
21 ) to reduce selection bias. Clinical and expenditure information is available in MEPS both from household respondents and from provider and pharmacy follow-back surveys. Both youths and adults were included in the analysis.
The study years were selected by necessity from those available in the MEPS: 1996 was the earliest year available, and 2005 was the most recent year when this study was undertaken. Because of the relatively small sample of antipsychotic users, we pooled adjacent years for this analysis and compared 1996–1997 with 2004–2005 (
22,
23 ). Thus means and proportions represent averages across each two-year period. The period 1996–1997 is early in the diffusion path of second-generation agents and is therefore an interesting comparator to the 2004–2005 period when these agents were widely used. Clozapine was approved by the FDA for the treatment of schizophrenia in 1989, risperidone in 1993, olanzapine in 1996, and quetiapine in 1997; other second-generation agents and newer approved indications for existing agents followed in the 2000s. Because relatively small subsamples remained for some analyses, we indicate cell sizes relying on unweighted samples of less than 100 respondents (
23 ) and urge caution in interpreting estimates based on small cell sizes. We retained the estimates in these cells for descriptive purposes only and did not conduct statistical tests against cells with fewer than 50 observations.
The prevalence of antipsychotic medication use was estimated from the MEPS prescription drug files in each year. Antipsychotic medications were identified via the Multum Lexicon categories appended by MEPS staff to each prescription (
24 ). Modifications to this system were made—for example, we excluded lithium, chlordiazepoxide, and prochlorperazine prescriptions from the Multum antipsychotic category.
Prescriptions were converted to defined daily dose (DDD) units in order to calculate quantity of use across formulations. The DDD system is promoted by the World Health Organization (www.whocc.no/atcddd) and allows the quantity of medications received to be expressed in terms of the number of days' supply of medication received on a standardized maintenance dose; these standardized doses were within the ranges specified in the CATIE protocol (
17 ). [A table showing the DDD for antipsychotics mentioned in the MEPS data is available as an online supplement at ps.psychiatryonline.org.] The conversion to DDD was applied to all antipsychotic prescriptions, regardless of target condition or age of the patient. Although the maintenance dose may not be the appropriate dose of medication for some antipsychotic users (for example, persons initiating therapy, patients who were youths or elderly, and off-label users), it does provide a way of examining average dosing over time across antipsychotic regimens. Increases in average DDDs per user may be due to actual dose increases or to polypharmacy, because doses are added up across formulations for each user. In contrast, decreases in average DDDs may be due to expansions in populations that may have lower target dosing (for example, patients who were youths or elderly), as well as increases in off-label use.
Other characteristics reported were obtained from the MEPS household survey (
20 ) for each year for antipsychotic users only. The reported characteristics focus on exogenous factors that describe the population of antipsychotic users, so as to avoid providing outcome data that may be influenced by choice of antipsychotic agent. We do, however, report antipsychotic and total health care expenditures and insurance variables, noting that these measures may be a function of treatment selection. Other characteristics examined include age, especially use among youths (that is, those younger than 18 years) and elderly persons (that is, those aged 65 years and older), payer status, employment status, and the number of other medication classes used. All spending estimates are presented in 2005 dollars, having been deflated by the gross domestic product deflator. Complex sampling weights and variances appropriate for the pooled analysis (
22 ) were used to obtain nationally representative estimates; differences across years were examined with t tests and chi square tests. All analyses were conducted in Stata 10 by using svy commands to adjust for the complex survey design of MEPS. Linearized standard errors are reported in the tables.
The issue of off-label prescribing of antipsychotics is only partially addressed in the analysis presented here. Medical conditions available in the household component of the MEPS are by self-report only and are available in the public release version of MEPS as collapsed three-digit ICD-9 codes to protect respondent confidentiality. Conditions are reported in the MEPS both in a separate section on medical conditions and injuries and from all reports of health services and medications used. We identified individuals who self-reported any condition in the ICD-9 categories for on-label use, namely schizophrenic disorders (295) or bipolar disorder, which is a subset of the larger ICD-9 category of affective disorders (296). However, because unipolar major depression is also classified in the ICD-9 category of 296, we were unable to separate off-label use for depression from on-label use for bipolar disorder, because of the unavailability of the fourth digit of the ICD-9 code that clarified whether the prescription was for bipolar disorder or depression. Therefore, our analysis will overcount on-label use. Our use of the terms on-label and off-label relies on these collapsed three-digit self-reported medical conditions and should be interpreted accordingly.
We also examined changes in other reported off-label uses of antipsychotics (
25 ) for anxiety spectrum disorders (
ICD-9 category 300), including obsessive-compulsive disorders. This category does not include other common anxiety disorders. We were unable to evaluate other off-label conditions that have been noted in the literature because of inadequate sample sizes in all years. Because of the potential for comorbidity among these three conditions, we indicated affective disorders only among those without reported schizophrenia, and we indicated anxiety spectrum disorders only among those without either schizophrenia or affective disorders. The reliance on self-reported conditions will potentially undercount each of these related conditions, while the use of collapsed three-digit
ICD-9 categories will overcount the more narrowly identified disease areas; the net effect is ambiguous. Our use of code groupings is not all-inclusive and was intended to identify major diagnostic categories for which antipsychotics might be used. There is no reason to believe that the population rate of underreporting or of actual disease prevalence has changed over the study period (
26 ).
Finally, we examined whether the characteristics that predict antipsychotic use and second-generation use among antipsychotic users have changed over time through a weighted linear probability model regression analysis, by using demographics, insurance status, year indicators, and interactions of these factors and year indicators. We ran this regression separately for adults and children, and we adjusted standard errors for heteroskedasticity and clustering.
Results
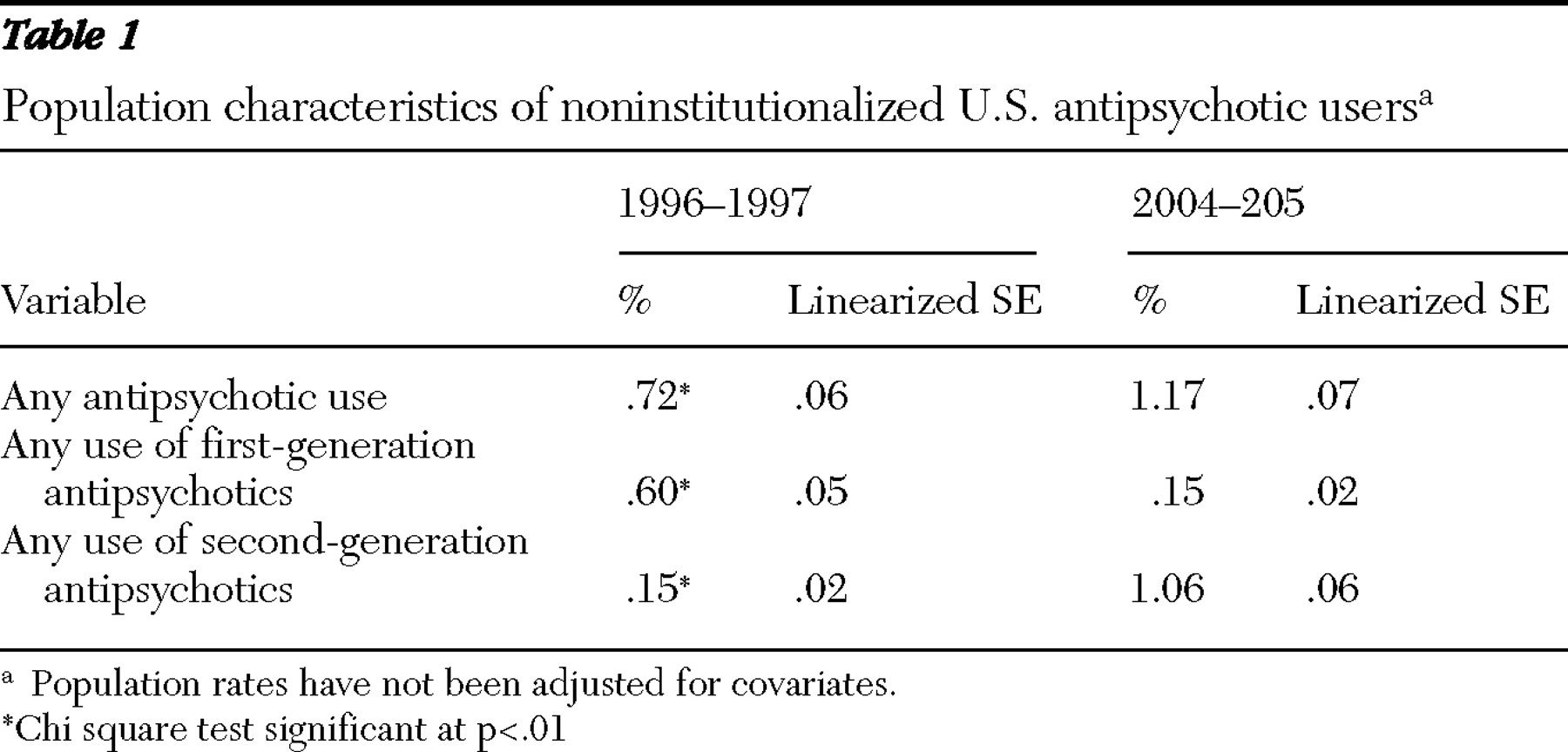
Second-generation antipsychotics diffused rapidly between 1996 and 2005. In 2004–2005, 1.17% of the noninstitutionalized U.S. population filled prescriptions for antipsychotic medications, up from .72% in 1996–1997 (p<.01) (
Table 1 ).
The population prevalence of second-generation use increased almost sevenfold in eight years; .15% of the population used second-generation antipsychotics in 1996–1997, and 1.06% used them in 2004–2005 (p<.01). The use of first-generation agents dropped during this period, from .60% to .15% of the U.S. population (p<.01).
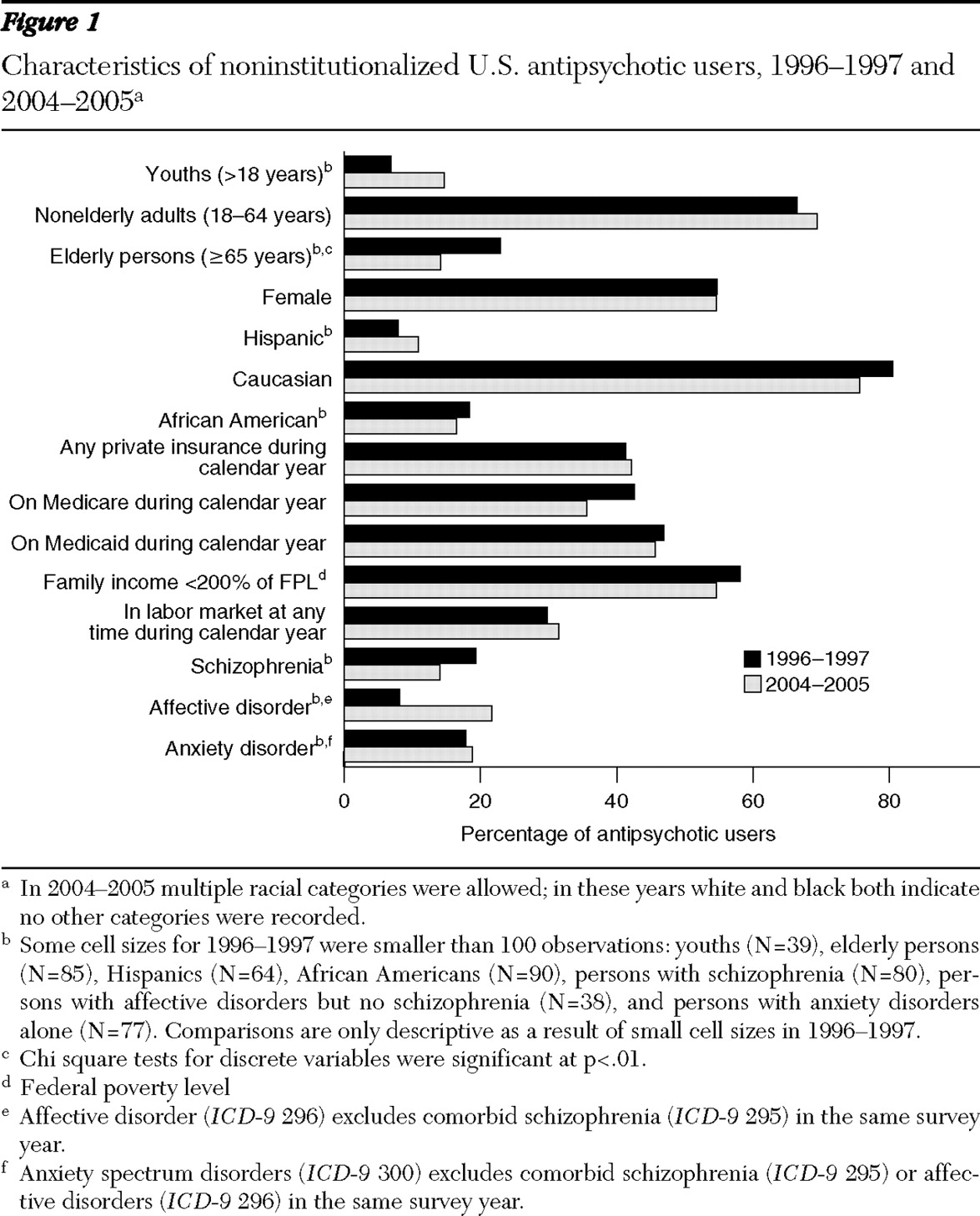
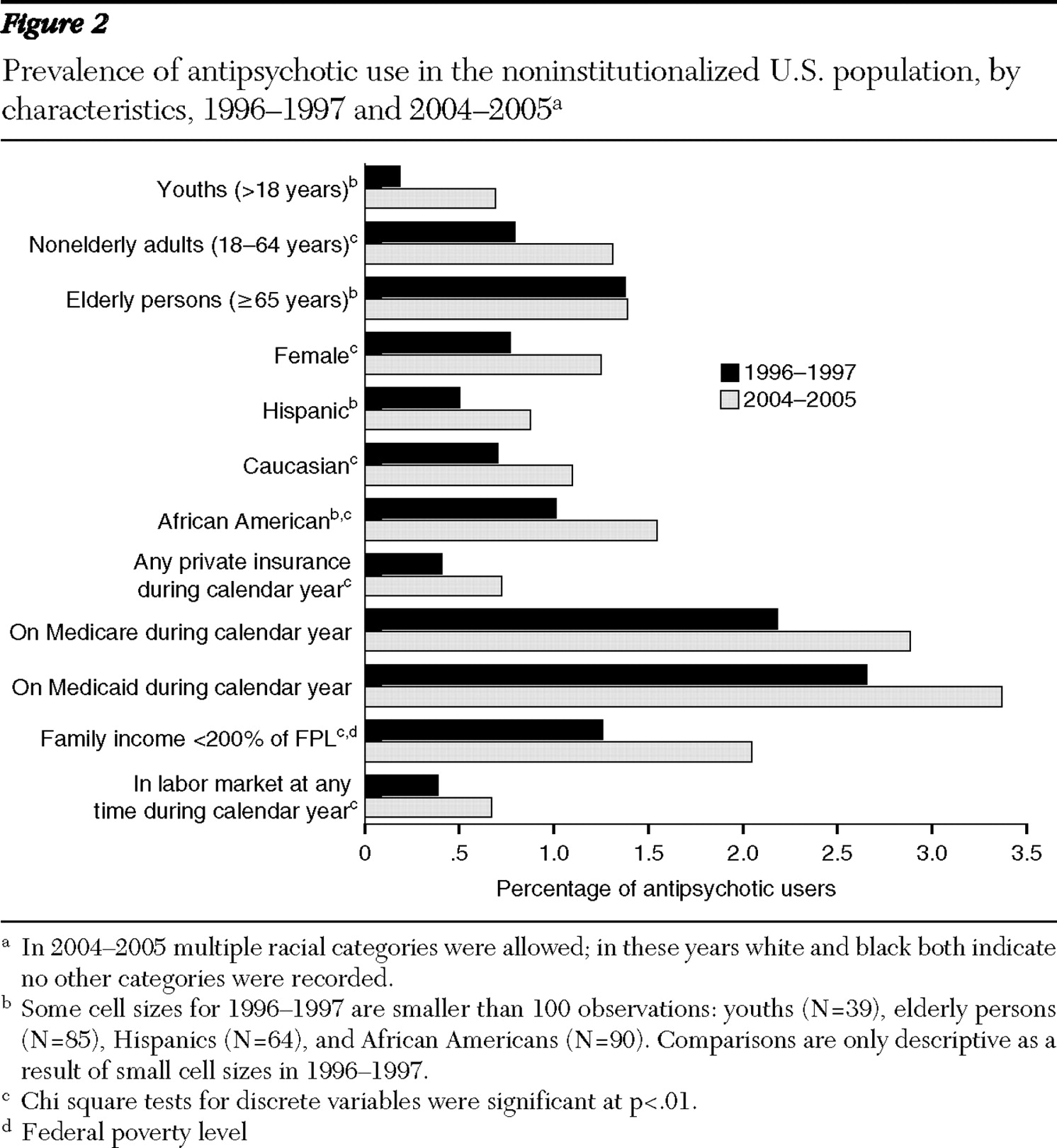
Although the size of the antipsychotic market has increased, the characteristics of users have remained fairly constant, with some exceptions. Notably, the average age of antipsychotic users has declined, from 49 years to 43 years (p<.01). This age reduction resulted from a shift in the distribution of users from elderly users toward youths (
Figure 1 ). [A table showing characteristics of antipsychotic users and rates of antipsychotic use is available as an online supplement at ps.psychiatryonline. org.] The percentage of the user population accounted for by youths has doubled for this eight-year period, from 7% to 15% of all users. This increase in antipsychotic use by youths can also be seen by a more than tripling of the rate of antipsychotic use, from .2% to .7% of youths in the U.S. population (
Figure 2 ). Elderly users declined from 23% of all users to 14%, but this group experienced no change in the rate of use. The rate of use among nonelderly adults also increased from .8% to 1.3%, although they remained a constant 67% to 69% of antipsychotic users. The gender, racial, and ethnic composition of users has been fairly stable over time, with women accounting for a slight majority of users (55%) and whites accounting for 76% to 81% of users.
The insurance status of antipsychotic users also remained fairly constant over this period. Although Medicaid is thought to dominate payments in this market, only 46% to 47% of outpatient antipsychotic users had Medicaid coverage during the periods examined. Antipsychotic users were almost as likely to be covered by private insurance, with 41% to 42% of the population of users covered by private plans during the calendar year. The labor market participation of individuals taking antipsychotics remained fairly constant at 30% to 32%, and the percentage of users with household incomes less than 200% of the poverty level remained fairly constant at 55% to 58%.
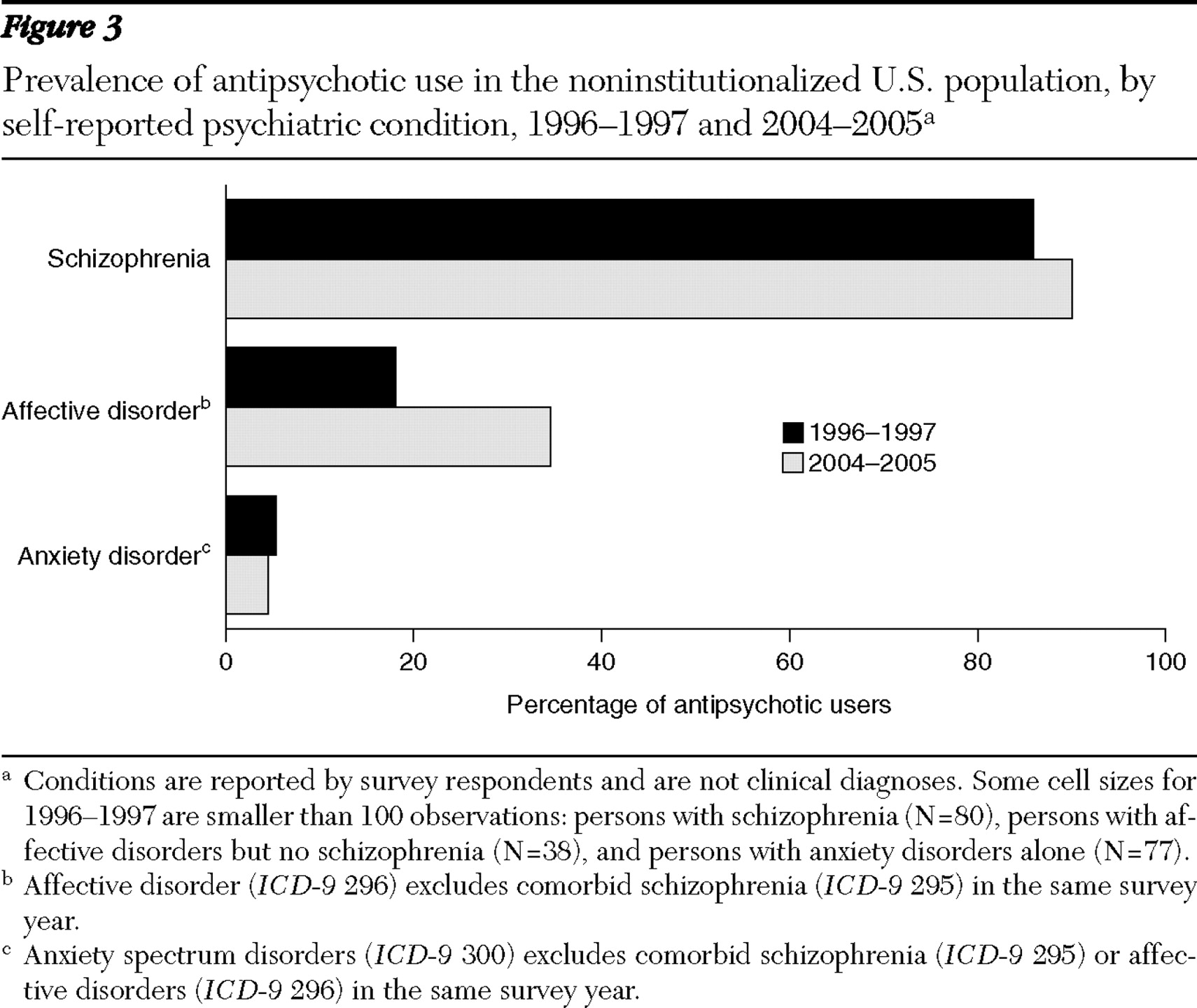
The data show a remarkable shift in the related on-label and off-label diagnoses for antipsychotic users (
Figures 1 and
3 ). Across the study period, 14% to 19% of users reported having a schizophrenia disorder, and those with this disorder reported a stable level of antipsychotic use (86% to 90%). However, the percentage of users with an affective disorder without comorbid schizophrenia increased substantially from 8% of all users to 22% of all users, and the rate of use for individuals in this category increased from 18% to 35%. The reported prevalence of anxiety spectrum disorders without schizophrenia or affective disorder (on-label comorbid conditions) among antipsychotic users was constant at 18%–19%, and the rate of antipsychotic use in this category remained constant at 5%. The self-reported prevalence of anxiety spectrum disorders without schizophrenia in the full U.S. population doubled from 2.4% to 4.8% during the study period (p<.01).
The average dosing of antipsychotics among users, expressed in DDD units, remained fairly constant across this period, ranging from 144 DDD units per user in 1996–1997 to 115 DDD units per user in 2004–2005. The average number of antipsychotic prescriptions per user remained constant, at about seven per year. The average antipsychotic user received a greater number of other medications, increasing from 3.3 in 1996–1997 to 4.1 in 2004–2005 (p<.05).
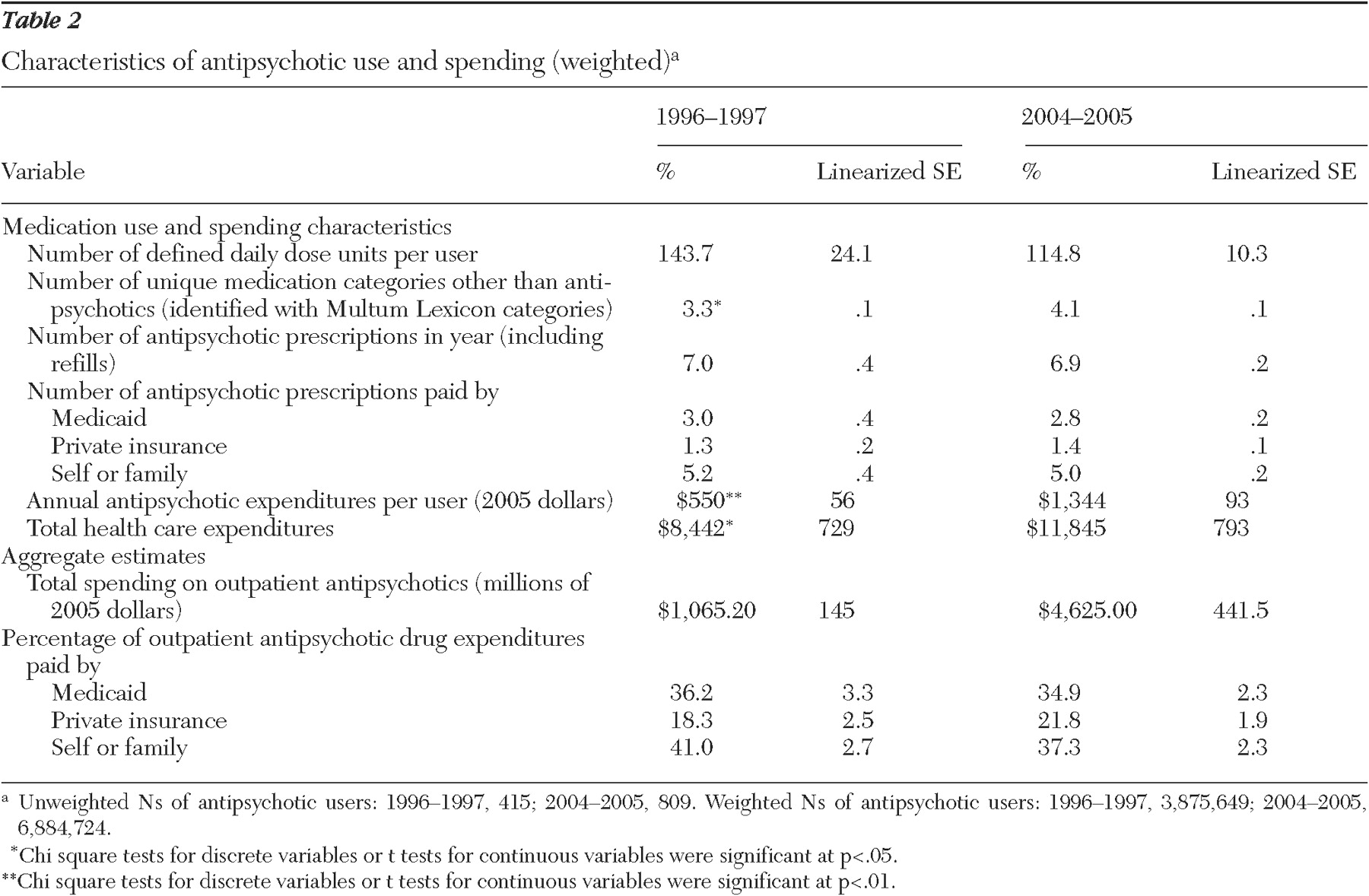
Although spending on antipsychotic medication in noninstitutional settings before applicable discounts has clearly increased over this period, starting as a $1.1 billion annual market in 1996–1997 (in 2004–2005 dollars) and over $4.6 billion per year in 2004–2005, the payer composition has remained stable (
Table 2 ). Individuals and their families are the largest source of payments for antipsychotics, paying for 37% to 41% of the total market, whereas Medicaid paid for just over one-third (35% to 36%). Private insurance payments accounted for about one-fifth (18% to 22%) of the outpatient antipsychotic market.
After controlling for covariates among adults, we found no difference across racial and ethnic categories in the probability of use of antipsychotic medication. Adults with self-reported schizophrenia, affective disorders, or anxiety spectrum disorders were more likely to use antipsychotics than were those without these conditions. Having an affective disorder without comorbid schizophrenia was 14 percentage points more likely to lead to an antipsychotic prescription in 2004–2005 than in 1996–1997 (p=.01). (For example, a nonelderly employed woman with private insurance and an affective disorder without schizophrenia had a 16.5% probability of receiving an antipsychotic medication in 1996–1997 and a 31.0% probability in 2004–2005.) No other condition or characteristic was associated with greater use in 2004–2005. Among youths in our sample, girls, African Americans, and Latinos were less likely than their respective comparison groups to use antipsychotics in all study years (p<.05), but the differences were small. Both schizophrenia and anxiety spectrum disorders, but not affective disorders, increased the probability of childhood antipsychotic use in 2004–2005 over 1996–1997.
Individuals receiving antipsychotic medications were 68 percentage points more likely to receive a second-generation antipsychotic in 2004–2005 than in 1996–1997. (For example, a woman on Medicaid with income under 200% of the federal poverty level who used an antipsychotic had a 23.5% probability of receiving a second-generation antipsychotic in 1996–1997 and a 91.3% probability of receiving a second generation antipsychotic in 2004-2005.) Respondents with income under 200% of the federal poverty level were more likely than those with higher income to receive a newer antipsychotic in 1996–1997, after the analyses controlled for self-reported conditions and other covariates, but they were less likely to receive a second-generation antipsychotic in 2004–2005. This suggests that low-income patients (or their doctors) were early adopters and that the other users caught up in 2004–2005. No other differences in predictors of second-generation use were significant at the 95% level between sample periods (full results available from author by request).
Discussion and conclusions
Despite a fairly constant prevalence rate of mental illness in the United States (
26 ), there have been notable shifts in the population of noninstitutionalized U.S. civilians using antipsychotic medications. We found substantial increases in the number of individuals filling antipsychotic prescriptions in the general U.S. population, but we did not find any increase in the level of use among antipsychotic users since the mid-1990s, as measured by both DDD units and the number of prescriptions.
The relatively constant rate of use among individuals who self-reported schizophrenia indicates that the rapid diffusion of second-generation antipsychotics was not associated with a greater rate of treatment in this population. Rates of antipsychotic use among persons reporting schizophrenia were stable across years, and no difference was found in reporting rates across years, although rates were lower than those in other studies (
27 ). The expansion of the antipsychotic market, instead, occurred among other disease categories, such as affective disorders.
The fact that the increased use of second-generation antipsychotics was not accompanied by a higher dosing is surprising and may mask several competing trends. Second-generation antipsychotics were originally touted as less toxic alternatives to first-generation agents (
28,
29,
30 ), which, if true, should have increased the level of use. However, during the sample period, more information became available on the negative health effects of some second-generation antipsychotic medications, leading the FDA in 2003 to issue a warning regarding the risk of hyperglycemia and diabetes mellitus based on several studies that were published during the study period reported here (
31 ). In addition, the remarkable increases in the rates of use for off-label conditions and use among youths (
5,
19,
25,
32,
33 ) may lead to lower dosing.
The data examined here largely predate the CATIE results, but the question remains, why did second-generation antipsychotics diffuse so rapidly? The answer is complex and is likely a function of factors not examined here, such as provider and patient expectations and preferences, drug company marketing effects (
12,
13 ), and the dominance of industry-funded trials in the early efficacy literature on second-generation antipsychotics (
28,
29,
30 ). In addition, clinical and malpractice risk concerns about the higher rate of tardive dyskinesia associated with first-generation antipsychotics also drove diffusion of second-generation antipsychotics. The trends here mimic the rapid diffusion of the newer antidepressant medications, especially among youths (
34 ).
It is clear that the appropriate use of antipsychotic medications improves the quality of life of people with schizophrenia and related disorders (
12 ), but recent evidence from CATIE and other studies (
35,
36,
37 ) has brought into question whether the more expensive second-generation antipsychotics are really a better use of resources, compared with first-generation antipsychotics. The lower risk of tardive dyskinesia may be one area in which second-generation antipsychotics have an advantage over first-generation antipsychotics, although this finding has recently been questioned (
35 ) and may be overshadowed by the greater risk of diabetes, obesity, and other related conditions (
4 ). Many of these risks were disclosed during the study period reported here (
38 ). Second-generation antipsychotics will only increase in use as their patents begin to expire, opening the market for less expensive generic substitutes and triggering a wave of less restrictive policies, especially in Medicaid and Medicare Part D formularies.
The greater use of antipsychotics in pediatric populations in this study is consistent with other literature (
5 ) and is an important policy issue. Antipsychotics are used in childhood disorders other than schizophrenia, such as autism and disruptive behavior disorders, but little is known about their efficacy and long-term effects, and differences in the types and intensity of side effects have been noted (
5,
7,
8 ).
A number of limitations should be noted for this analysis. The MEPS does not survey institutionalized individuals, who may have different patterns of antipsychotic use. Therefore, the size of the full antipsychotic market and specifically the amount paid by Medicaid are understated in this study. Several of the cells for 1996–1997 are based on fewer than 100 observations, and these data may be less reliable; they are presented for descriptive purposes only but this in itself is a telling indicator of the rates of use in these subpopulations in the earlier period. The self-reported medical conditions are not as accurate as clinician diagnoses and clearly understate the prevalence of disease in the population. For example, Wu and colleagues (27) estimated a 12-month prevalence of schizophrenia of .51% using a variety of claims data sources, whereas the MEPS-reported prevalence is .16 to .18%. In addition, the on-label rates reported here, ranging from 27.5% in 1996–1997 to 35.8% in 2004–2005, are likely too low, although the inclusion of all affective disorders with ICD-9 code 296 as on-label use underestimates off-label use.
Other rates for off-label antipsychotic use reported in the literature are based on claims diagnoses and are slightly higher than those found in the study reported here. Rates for other studies ranged from 36% in Georgia Medicaid in 2001 (
39 ) to 44% of second-generation antipsychotic users in the United States in 2004 (
40 ). Radley and colleagues (
9 ) reported an off-label rate of 31% for the broader psychotropic category. The conversion to DDD units is based on current dosing guidelines, and the conversion did not vary over the study period. We did, however, find consistent results whether we looked at the annual number of DDD units or the number of antipsychotic prescriptions.
Although literature from CATIE has increased the knowledge base surrounding the use of antipsychotic medications, there are a number of issues that remain to be examined. As with most clinical trials, the population studied in CATIE may not resemble the full spectrum of medication users (
13 ). In particular, users with schizophrenia and Alzheimer's disease represent a small fraction (<20%) of all antipsychotic users. Further information on the cost-effectiveness of antipsychotic use in nontraditional but increasingly common categories of use would inform the diffusion process. Finally, although CATIE itself may affect antipsychotic diffusion, early results in a privately insured population found no effect of CATIE on antipsychotic use (
41 ). Inferences from CATIE were complicated by the introduction of Medicare Part D shortly after the initial CATIE results were released; Part D itself will also substantially affect the use of antipsychotic medications as Medicaid funding and associated restrictions on medication use for dually eligible Medicare beneficiaries were shifted to the Medicare program (
42 ). If prescribers become willing to return to the earlier technology of first-generation antipsychotics as the CATIE message is repeated, we may see a substantially different diffusion process over the next decade.
Acknowledgments and disclosures
CATIE was supported by grant N01-MH-90001 from the National Institute of Mental Health (NIMH). A list of study locations and principal investigators can be found at www.catie.unc.edu/schizophrenia/locations.html. Support for the study reported here was received by Dr. Domino under grant K01-MH-065639 from NIMH. The authors appreciate the excellent comments by Richard Frank, Ph.D., and the SPRINT group at the University of North Carolina.
Dr. Swartz has been a consultant or speaker for or has received research funding from Bristol-Myers Squibb, Eli Lilly and Company, and Pfizer. Dr. Domino reports no competing interests.