Reports from the United States indicate that the use of antipsychotic drugs among children and adolescents is increasing (
1,
2,
3,
4,
5 ). Second-generation antipsychotics were introduced early in the 1990s and characterized by a supposedly smaller potential for inducing extrapyramidal side effects (
6 ). Use of second-generation antipsychotics among youths has increased in the United States, in contrast to a constant or even declining use of first-generation antipsychotics (
2,
7 ). Pharmacoepidemiological data about antipsychotic drug use in other countries are scant. Available data about overall antipsychotic use among children from European countries show no change over recent years in Italy until 2004 (
8 ) and a small increase in the Netherlands until 2002 (
9,
10 ).
Second-generation antipsychotics are commonly prescribed by child psychiatrists in several countries, including the United Kingdom (
11,
12 ), Finland (
13 ), and Australia (
14 ). Since the introduction of this class of drugs, reports have appeared about their use for pervasive developmental disorders, affective disorders, and disruptive behavior disorders (
15 ). But in many countries, such as the Netherlands, the use of antipsychotic drugs to treat children is not approved, except for a few first-generation antipsychotic agents and indications (for example, haloperidol to treat Tourette's disorder and psychosis). Therefore, off-label use and, consequently, efficacy and safety issues of administering this type of drug to children are important to understand.
The available peer-reviewed studies describe either prevalence or incidence of antipsychotic drug use but not duration of use. However, duration of exposure is relevant for the evaluation of efficacy, safety, and tolerability. The aim of this study was to describe prevalence, incidence, and duration of antipsychotic drug use over the period 1997–2005 among youths from the Netherlands and whether changes are associated with specific age groups, gender, or class of antipsychotic drug (first or second generation).
Methods
Setting and study population
This study was performed with pharmacy dispensing data from the InterAction database (www.iadb.nl) (
16 ). The database comprises all prescription drug dispensing data since 1997 from about 500,000 persons in the northern and eastern parts of the Netherlands. It includes all prescriptions, regardless of prescriber, reimbursement status, or insurance, apart from over-the-counter drugs and drugs dispensed during hospital stays. Youths who ranged in age from infancy through 19 years were selected from the database. Their number (denominator) was 95,158 in 1997, 112,131 in 2001, and 119,612 in 2005.
Study variables and analysis
Antipsychotic drugs were defined with the World Health Organization's Anatomical Therapeutic Chemical/ Defined Daily Dose classification system as the members of class N05A, except N05AN (lithium). Clozapine, olanzapine, quetiapine, sulpiride, risperidone, aripiprazole, and ziprasidone were considered second-generation antipsychotic drugs. The remaining drugs of class N05A were considered first-generation antipsychotic drugs (for example, haloperidol, pimozide, pipamperone, perphenazine, and thioridazine).
Antipsychotic drug users (numerator) were youths from infancy through age 19 years who received at least one antipsychotic prescription between January 1, 1997, and December 31, 2005. Prevalence of antipsychotic drug use was estimated per year and was defined as the number of youths for whom any antipsychotic was prescribed per thousand youths. Incidence was defined analogously. A youth was considered a new first-time user, or an "incidental user," if that youth's data were present in the database for at least one year and the individual was receiving a prescription for an antipsychotic for the first time. This prevented ongoing users who were moving into the catchment area of the database and users who were temporarily hospitalized from being seen as new users. Thus someone was counted as an incidental user on first-ever use of an antipsychotic drug. Prevalence and incidence were stratified by class of drug (second generation or first generation), gender, and age group (infant to four years, five to nine years, ten to 14 years, and 15 to 19 years). For incidence, absolute numbers were much smaller after stratification for gender, and we chose not to report those data. For brevity only data for 1997 (for incidence, 1998), 2001, and 2005 are reported.
Duration of antipsychotic drug use was described by means of a Kaplan-Meier estimator. An episode of antipsychotic drug use was considered to begin when a child became a new user (as described before). An episode ceased if, since the last prescription, at least the amount of days for which medication was prescribed plus 90 days had passed and the youth could still be followed in the database. All other cases were considered censored according to Kaplan-Meier analysis. Duration of use over the entire period 1997 to 2005 was also described after stratification for gender, class of drug, and age group. Duration of use in two time frames was compared across new first-time users in the years 1998–1999 and 2001–2002. For this comparison, cases from the cohort of 1998–1999 were censored after 2002 because the cohort of 2001–2002 could be followed only until the end of 2005.
Differences were considered significant at p<.05. Statistical analyses were performed with SPSS for Windows, version 13.0 (
17 ). The 95% confidence interval for the exposure rates was calculated with the score method, with continuity correction for small proportions (
18 ).
Results
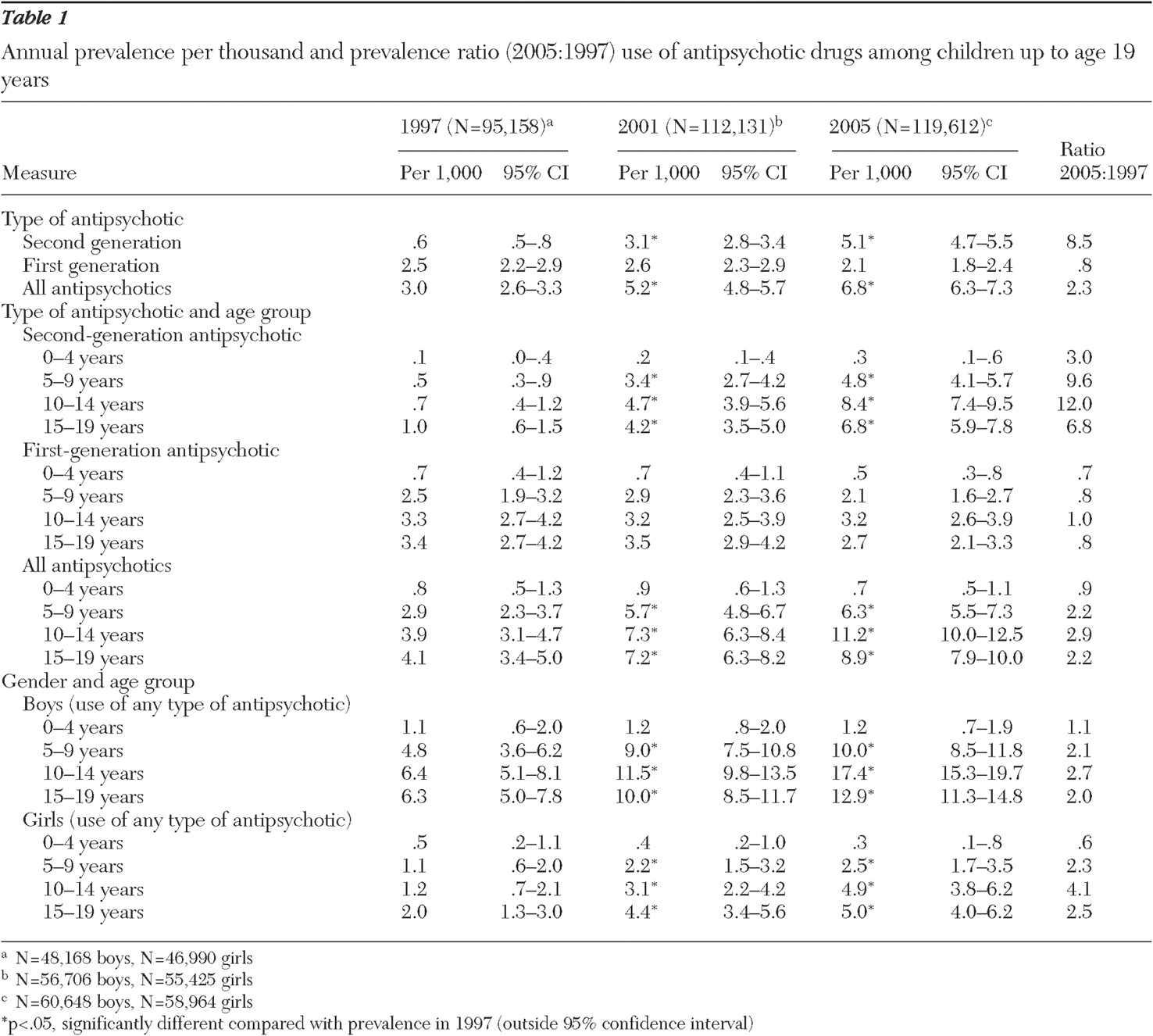
The prevalence of antipsychotic drug use for youths through 19 years of age increased from 3.0 to 6.8 per thousand from 1997 to 2005 (
Table 1 ). Marked differences between subgroups and the two classes of drugs were found. The use of second-generation antipsychotics increased almost ninefold (.6 to 5.1 per thousand), but use of first-generation antipsychotics remained relatively stable. The increase in use of second-generation antipsychotics was most pronounced for five- to nine-year-olds (almost tenfold) and for ten- to 14-year-olds (12-fold).
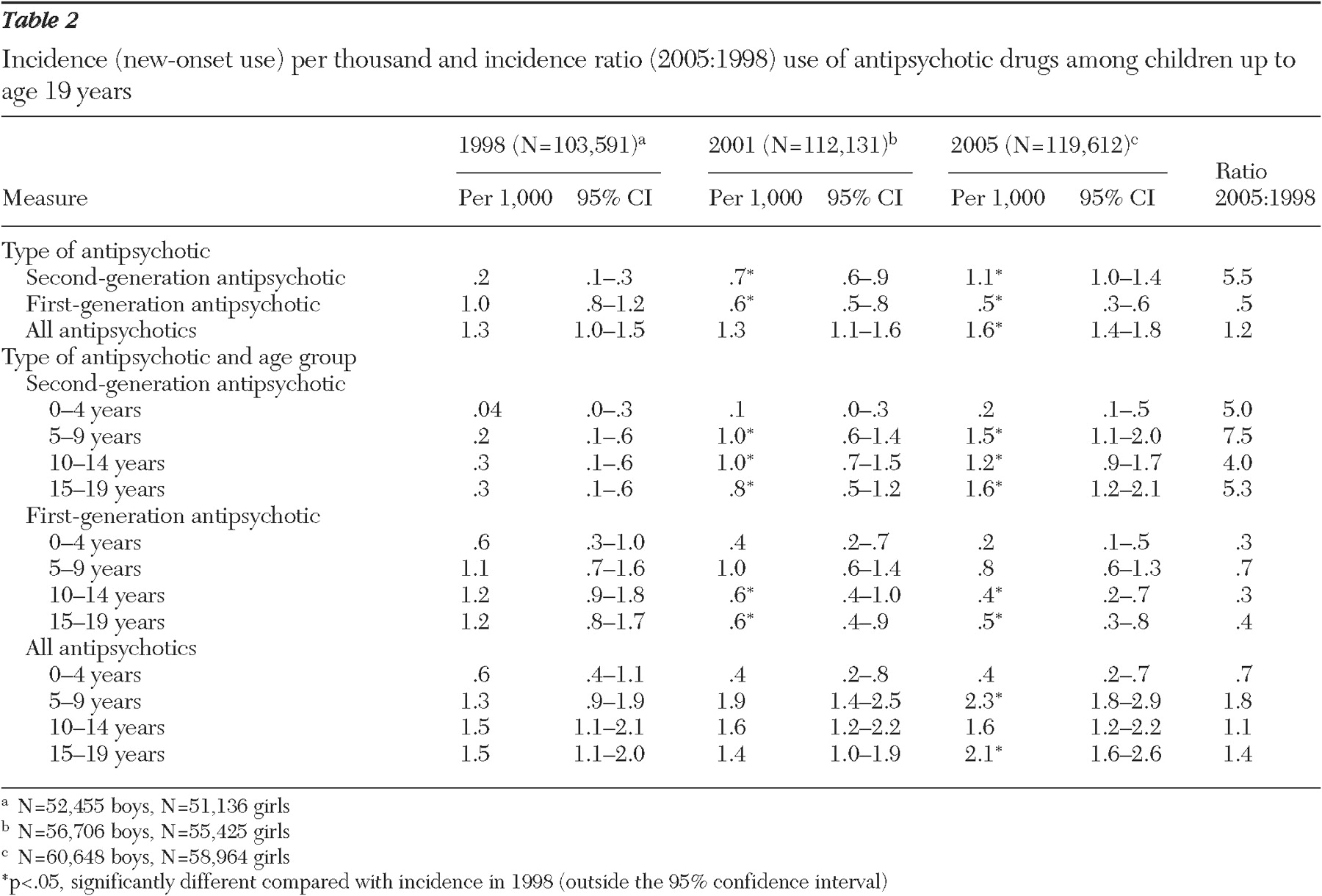
New-onset use (incidence) of second-generation antipsychotics increased more than fivefold from 1998 to 2005, with the biggest increase (7.5-fold) among five- to nine-year-olds (
Table 2 ). The incidence of first-generation antipsychotics was reduced by half over this period. New-onset use of first-generation and second-generation antipsychotics together increased modestly, from 1.3 per thousand in 1998 to 1.6 per thousand in 2005.
Boys used considerably more antipsychotic medications than girls. Over the years the ratio of boys to girls using antipsychotics remained stable at roughly 4 to 1 up to age 14 years and 2 to 1 in the oldest age group (15–19 years).
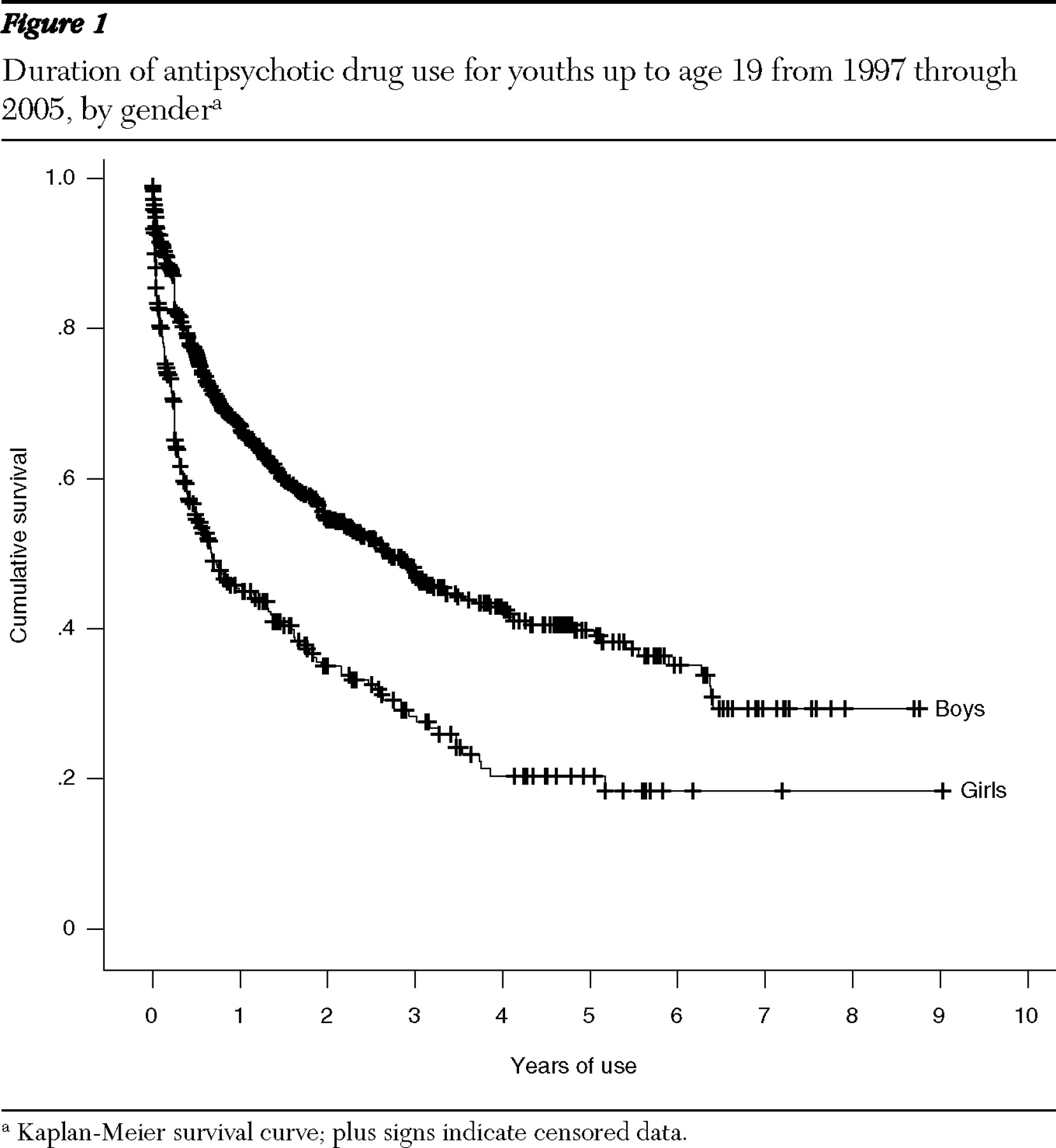
The median duration of use of antipsychotic drugs was 1.9 years but with notable differences between subgroups (
Table 3 ). The median duration of use for boys was 2.7 years, and for girls .7 year (
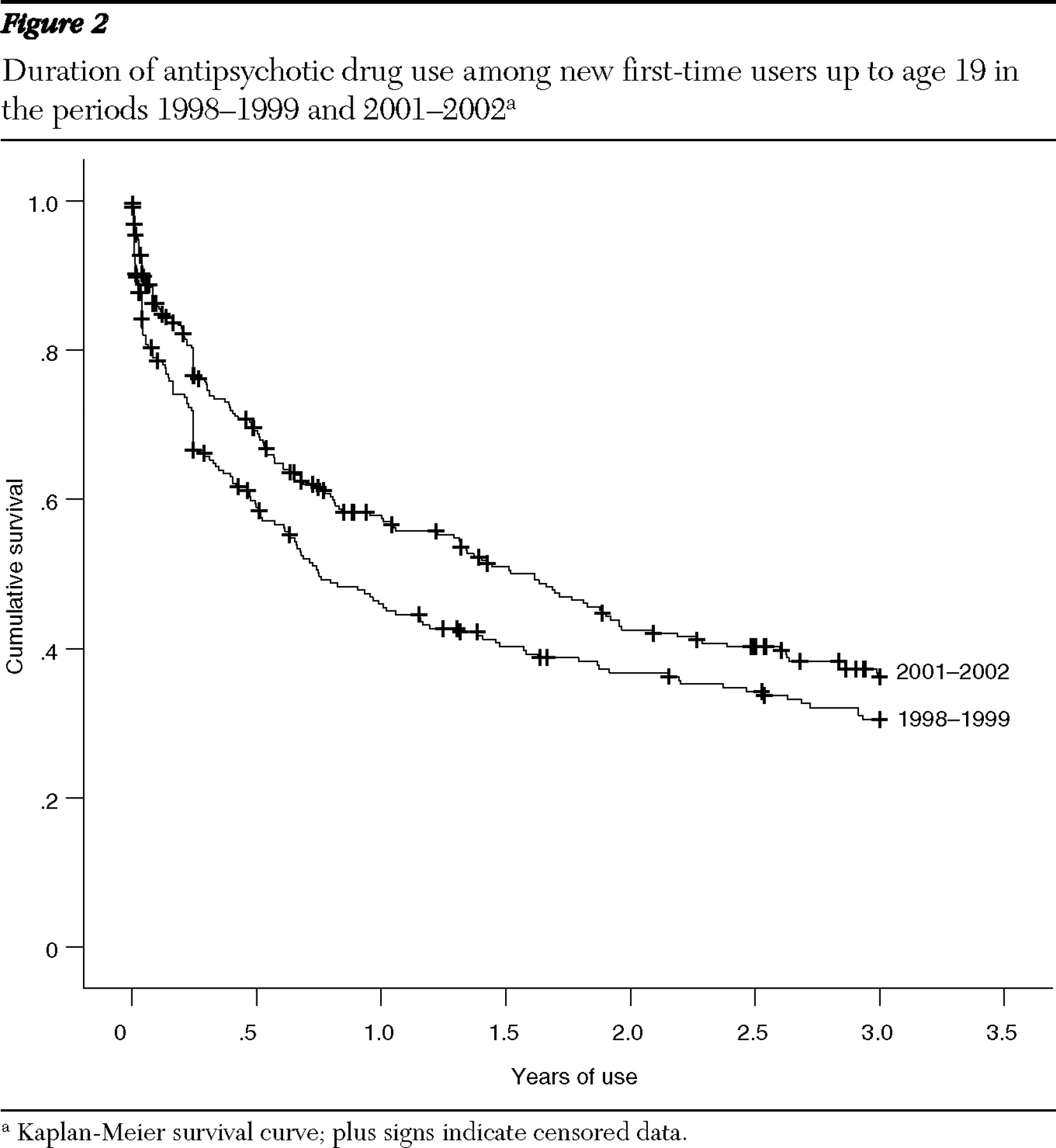
Figure 1 ). The median duration of use was longest for five- to nine-year-olds, at 5.1 years, and shortest for 15- to 19-year-olds, at .7 year. The median duration of use of second-generation antipsychotics was 2.9 years, 3.5 times as long as that of first-generation antipsychotics. The median duration of use for new users doubled from .8 year in 1998–1999 to 1.6 years in 2001–2002 (
Figure 2 ).
Discussion
Among youths in the Netherlands the prevalence of antipsychotic drug use has more than doubled over a period of nine years. This increase was mainly driven by a large increase (almost ninefold) in the use of second-generation antipsychotics. Also, new-onset use of second-generation antipsychotics increased fivefold, and they were used considerably longer than first-generation agents.
The findings for some subgroups are worth mentioning. From 1997 to 2005, second-generation antipsychotic drugs were increasingly prescribed to five- to 14-year-olds. It can even be assumed that a considerable part of this age group had already started using antipsychotic drugs between the ages of five and nine years because incidence and duration of use were highest in this age group. In contrast the prevalence of antipsychotic drug use was somewhat lower among 15- to 19-year-olds, and median duration of use was only one-fifth of that of five- to nine-year-olds. No changes in prescription rates were found in the youngest group, infants to four-year-olds, with the exception of a significant shift toward the second-generation antipsychotics.
Boys used more antipsychotics and for longer periods. Also, there was a possible trend for increasing use of antipsychotics overall among ten- to 14-year-old girls.
The pattern that emerged was the use of more antipsychotics for longer periods among younger children, predominantly among boys. Several developments could explain this pattern. More children probably used mental health care in 2005 than in 1997, and this increase could be associated with an increase in overall use of antipsychotics (incidence and prevalence), but less so with an increased duration of use. A growth in referrals of 9% was documented for the period 2000–2002 in the Netherlands (
19 ), but data for our population and time frame were not available. The reluctance to prescribe psychotropic medication to children most likely decreased during this time frame (
11,
20 ).
Several factors may have led to the striking increase in the use of second-generation agents. In recent years marketing efforts were almost exclusively directed at these newer antipsychotics. The supposedly smaller potential for inducing extrapyramidal side effects and tardive dyskinesia by second-generation antipsychotics may have decreased the reluctance to prescribe these drugs to children (
3 ) and perhaps also for use over longer periods. Longer use of second-generation antipsychotics could be explained by their better tolerability compared with first-generation antipsychotics. This phenomenon has been observed among adult patients with a first psychosis (
21 ). Finally, the emergence of several new indications, especially for the second-generation antipsychotics—such as pervasive developmental disorder and aggression—may be an important factor in increasing use (
15 ). Some of those diagnoses, such as pervasive developmental disorder, begin earlier in life, are more prevalent among boys, and have a more continuous course than the previously established indications of psychosis and mania. Use of these agents for new indications may be associated with increased duration of use and use among younger children.
A comparison with databases from other countries and health care systems should be made with care. For example, the prescription dispension database that was used in this study is not restricted to one health insurance system, unlike databases in the United States. This characteristic has an influence on usage (higher use in Medicaid than in a managed care program) (
2 ).
A similar strong (sixfold or higher) increase in prescriptions for second-generation antipsychotics has been described in the United States (
2,
3,
4 ). Our finding in this study that the increase was especially striking for five- to 14-year-olds partially matches U.S. findings that the increase was strongest among five- to nine-year-olds (
2 ).
Prevalence of overall antipsychotic use (both classes) was quite similar to the most recent findings from a U.S. prescription claims study of several regions and two insurance plans (for 2001, between 3.4 and 15.5 users per thousand in the United States [2], compared with 5.2 users per thousand in the Netherlands). Prevalence among children in a prescription claims database from Italy was nearly half that of this Dutch population (2.91 children per thousand in 2004 [
8 ], compared with 6.8 per thousand in 2005 in the Netherlands). Use of antipsychotics to treat new onset of psychotic disorders in a managed care plan for a Tennessee population in 2001 was higher than in this study (1.3 prescriptions per thousand, compared with 4.5 per thousand in the United States) (
3 ). Similarly, in the United States use of all antipsychotics among youths grew by a factor of 2 or 3 from 1996 to 2001 (
2 ).
One U.S. study found that increase in new prescriptions of antipsychotics was most pronounced for adolescents (
3 ). Another U.S. study, which focused on mental health visits rather than on prescriptions as a data source, found no relation between age and the odds of receiving antipsychotic treatment (
4 ). A third U.S. study, of commercially insured children, found relatively higher rates of antipsychotic prevalence for prepubertal children (
5 ). Our study found the highest prevalence among ten- to 14-year-olds, but the strongest increase among five- to 14-year-olds suggests a similar trend toward use by younger children in the Netherlands. A higher prevalence of antipsychotic drug use among boys also has been described in the United States (
2,
5 ). This difference was even more pronounced for Dutch children, but we cannot offer an explanation for this finding. The comparison between data from the United States and the Netherlands demonstrates that the large differences found between the countries in the use of stimulants (
1,
9 ) and antidepressants (
22 ) were not found for antipsychotic drugs.
It is important to recognize the possible risks of exposing young patients to second-generation antipsychotics for considerable periods (much longer than in the available controlled trials) for new indications. Moreover, antipsychotic drug use among children for the indications mentioned above should be considered off label, at least in the Netherlands. Several important side effects of second-generation antipsychotics have been reported, including weight gain; metabolic disturbances, which even may lead to diabetes (
23 ); and effects through prolactin on the endocrine and reproductive system (
24,
25 ). It should be kept in mind that children might be at greater risk than adults of some of those side effects (
26 ).
Strengths, limitations, and implications of this study
This study was the first study to report about incidence and prevalence of antipsychotic drug use over a period of nearly a decade from a European country. To our knowledge it is the first report about duration of use, for subclasses of antipsychotic drugs and for different age groups.
This study had several limitations. For example, we examined prescriptions but not actual use. Dosage and dosing schedules may vary considerably but were not reported. Because we focused on a prescriptions dispension database, we could not report about indications for drug use and type of prescriber. Therefore, it was not possible to give more precise explanations for the observed changes. We did not study data for the Netherlands as a whole, but a part of the country, so it is not possible to predict the effect this had on the results, if any. The data for this study did not include antipsychotic drug use among inpatients (for example, institutionalized children with mental retardation), which is quite different from that of outpatients (
27 ). This fact could have led to an underestimation of prevalence and duration of use in the database (hospitalization interrupted a period of use). The observations in some age groups (for example ten- to 14-year-old girls) merit more detailed research by subtype of drugs.
Our definition of incidence (a new first-time user of any antipsychotic) had some implications. Incidental users in 1998 were traced back only to 1997, but new users in 2005 could be traced back for a longer period, resulting in a possible overestimation of incidence in earlier years. Episodic use by the same user was not reported, which may have skewed the results for duration of use in some of the comparisons. For example, duration of use was much shorter in the age group of 15- to 19-year-olds, and this may obscure intermittent use or frequent lapses in a treatment regimen that was intended to be continuous. Moreover, the oldest age group was somewhat more difficult to follow, because the age represented is the age at which some individuals leave home and move to other areas of the country.
Switching between subclasses of drugs (from first-generation to second-generation antipsychotics) and simultaneous prescribing were not measured separately. The difference between the sum of second-generation and first-generation antipsychotic drug use and total prevalence was an indication of the amount of switching plus simultaneous use within a year. This difference was never more than 10% of the sum of use of both classes of drugs and increased from .1 per thousand in 1997 to .5 per thousand in 2001 and .4 per thousand in 2005.
Research implications
The reasons for the described changes of antipsychotic drug use are unknown. Pharmacoepidemiological data combined with information about indications are therefore a very valuable addition to the knowledge base for psychiatric research. Our data from 2005 suggest that priority should be given to studies of second-generation antipsychotics that are conducted for at least one year. Research also should be focused on indications where evidence for efficacy is lacking and on side effects that unfold in the course of long-term treatment. Even newer antipsychotics (aripiprazole and quetiapine, for example) have been introduced, and long-term data about safety and efficacy are urgently needed before youths are exposed to them.